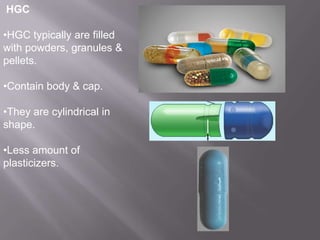
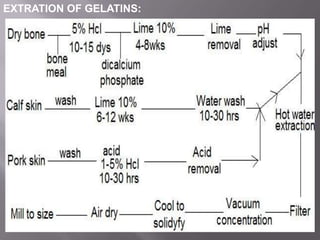
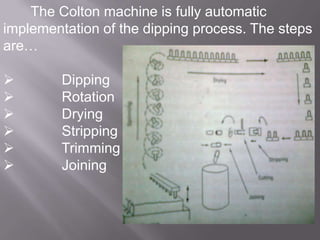
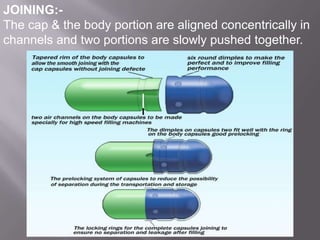
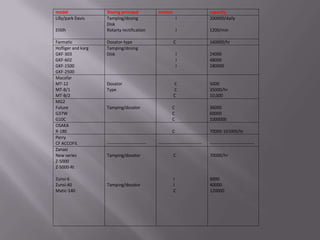
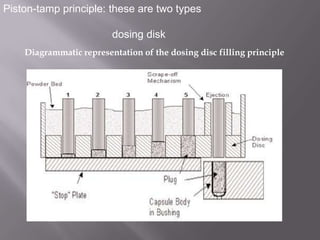
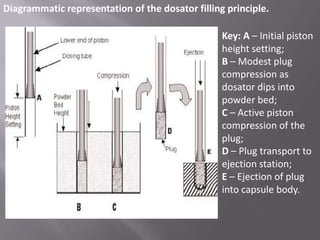
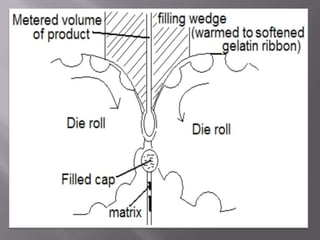
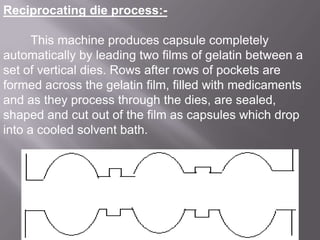
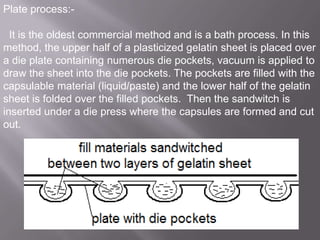
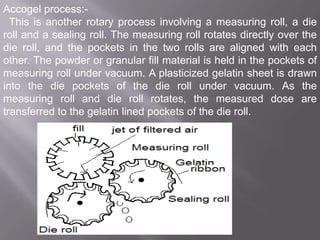
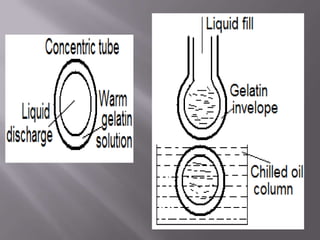
The document discusses hard and soft gelatin capsules, their definitions, advantages, disadvantages, and manufacturing processes. It explains the composition of the shell, materials used, and the steps involved in capsule formulation and filling. Additionally, it covers the importance of various additives and the equipment used in capsule production, highlighting key factors that influence capsule quality and efficacy.