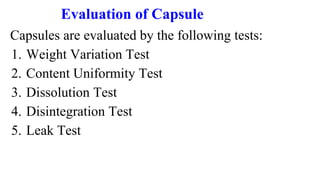
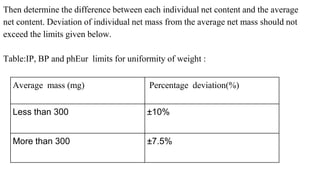
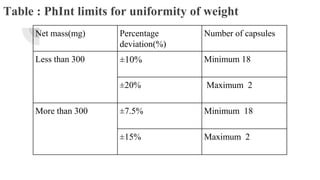
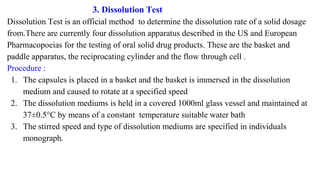
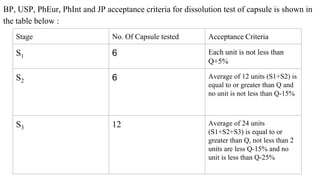
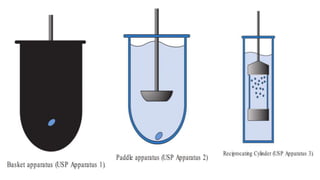
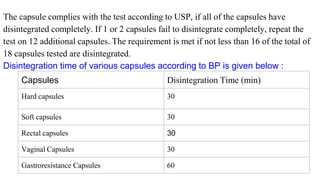
This document outlines the evaluation procedures for pharmaceutical capsules, including tests for weight variation, content uniformity, dissolution, disintegration, and leak testing. Each test is described in detail, specifying methods and acceptance criteria based on pharmacopoeial standards. The reference section lists various pharmaceutical sources and guidelines pertinent to the evaluation methods discussed.