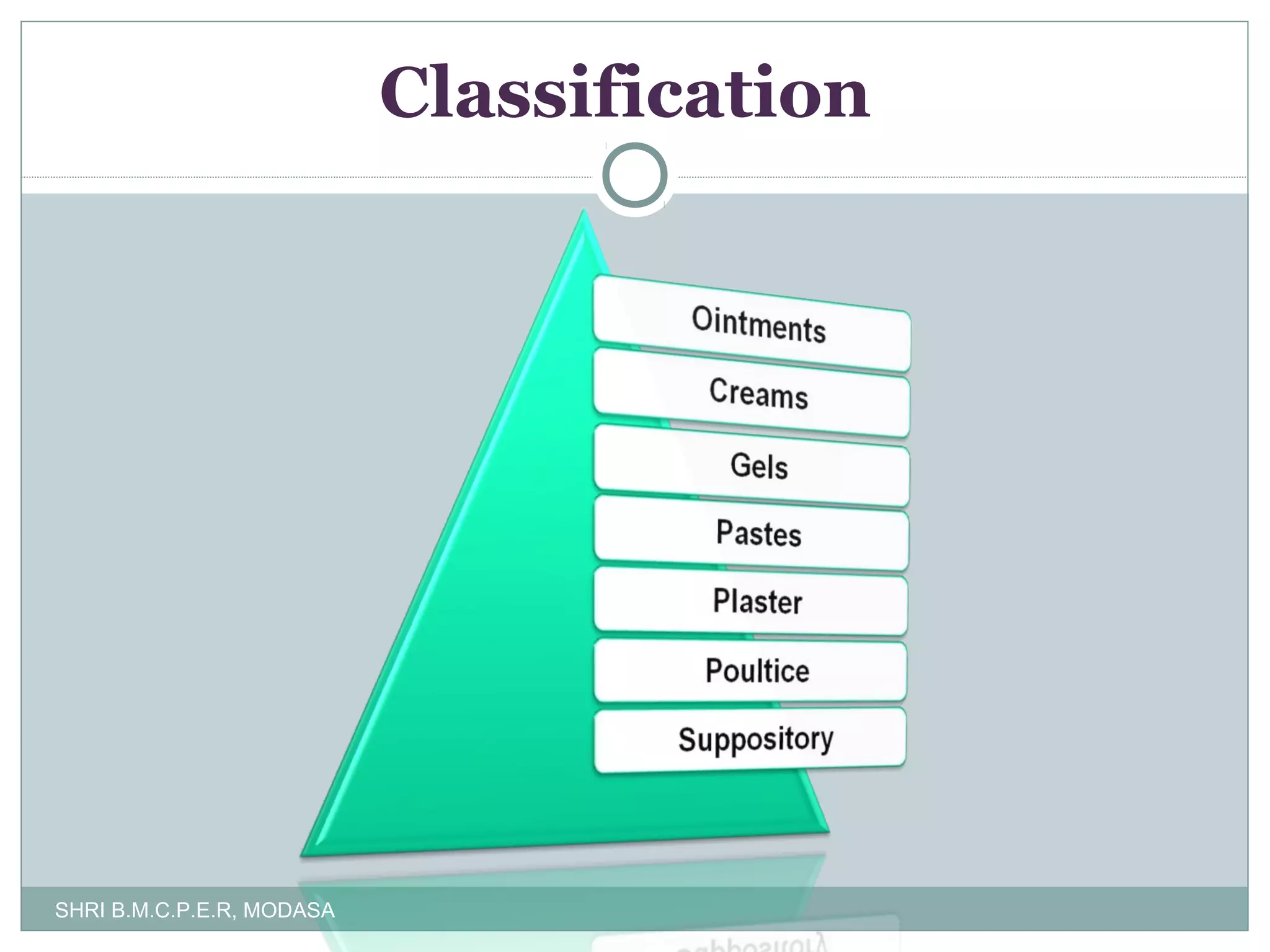
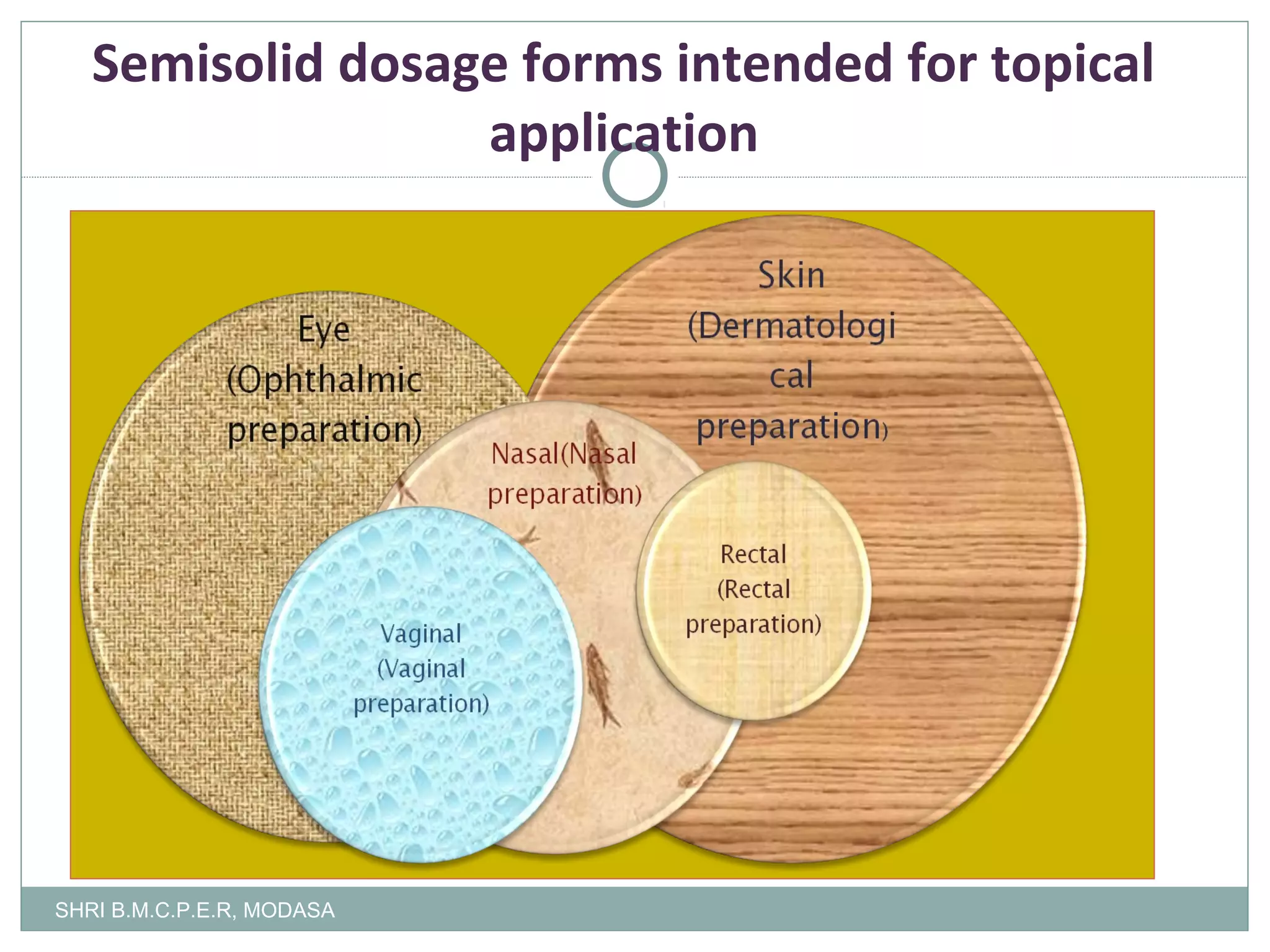
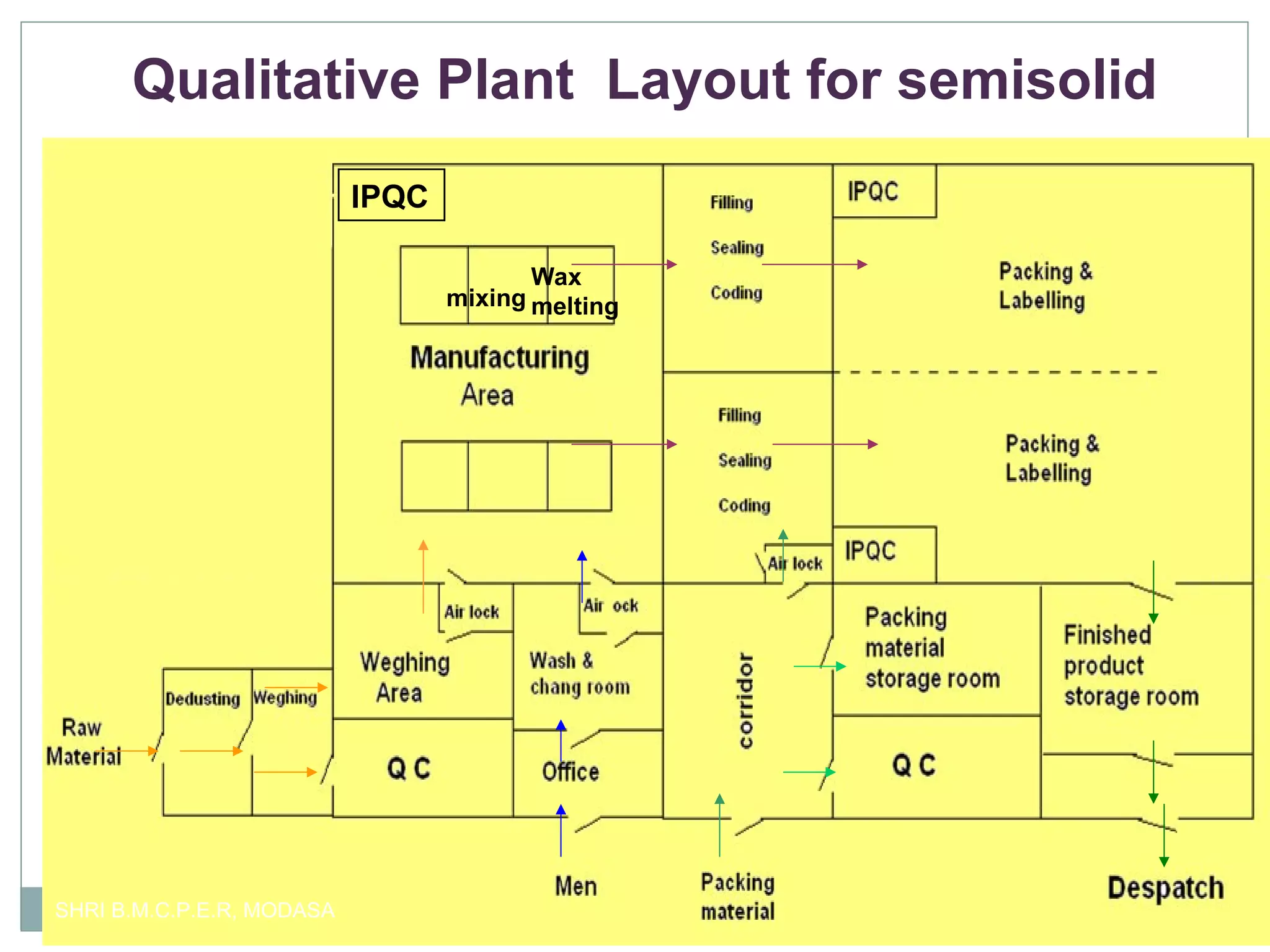
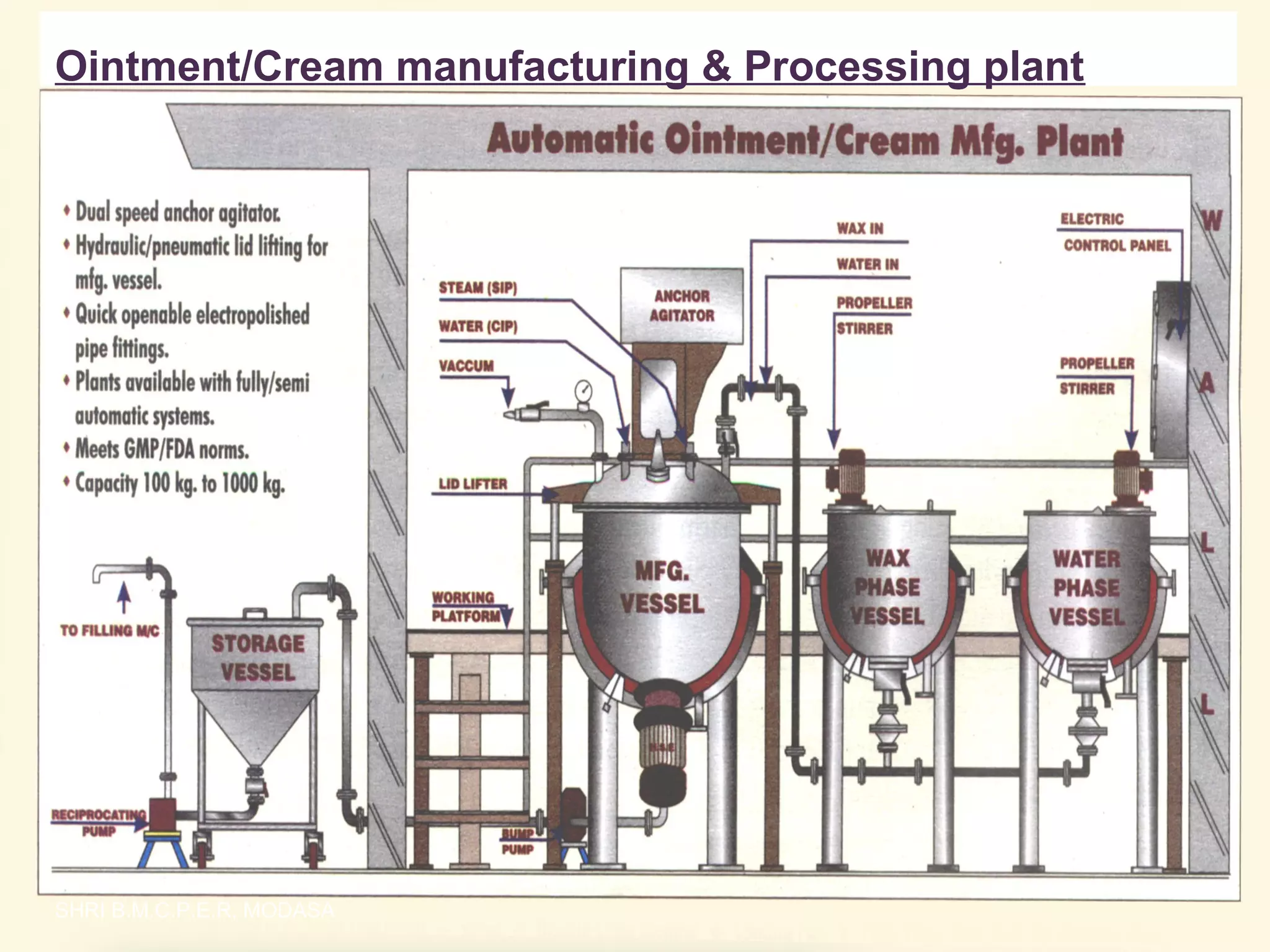
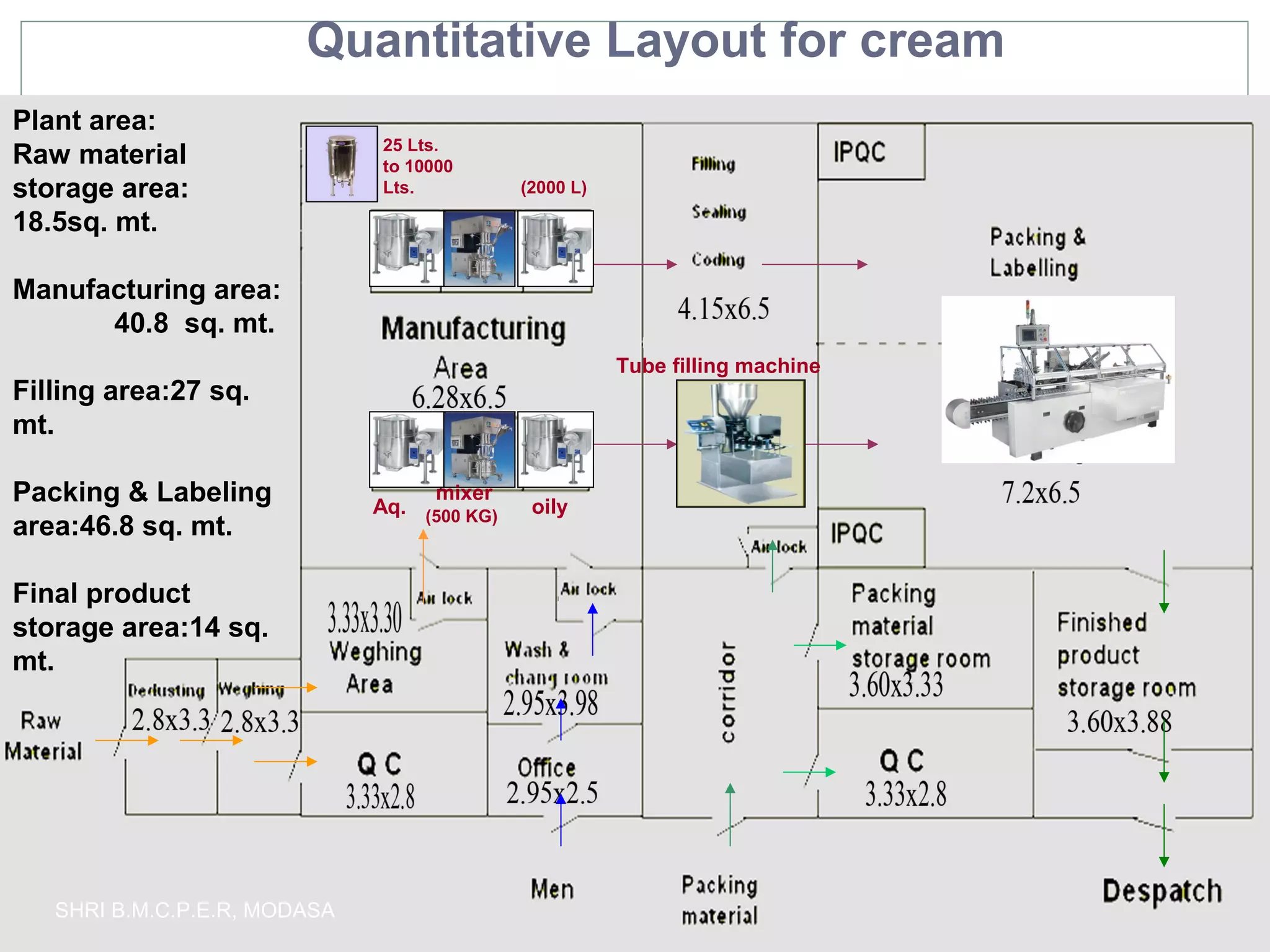
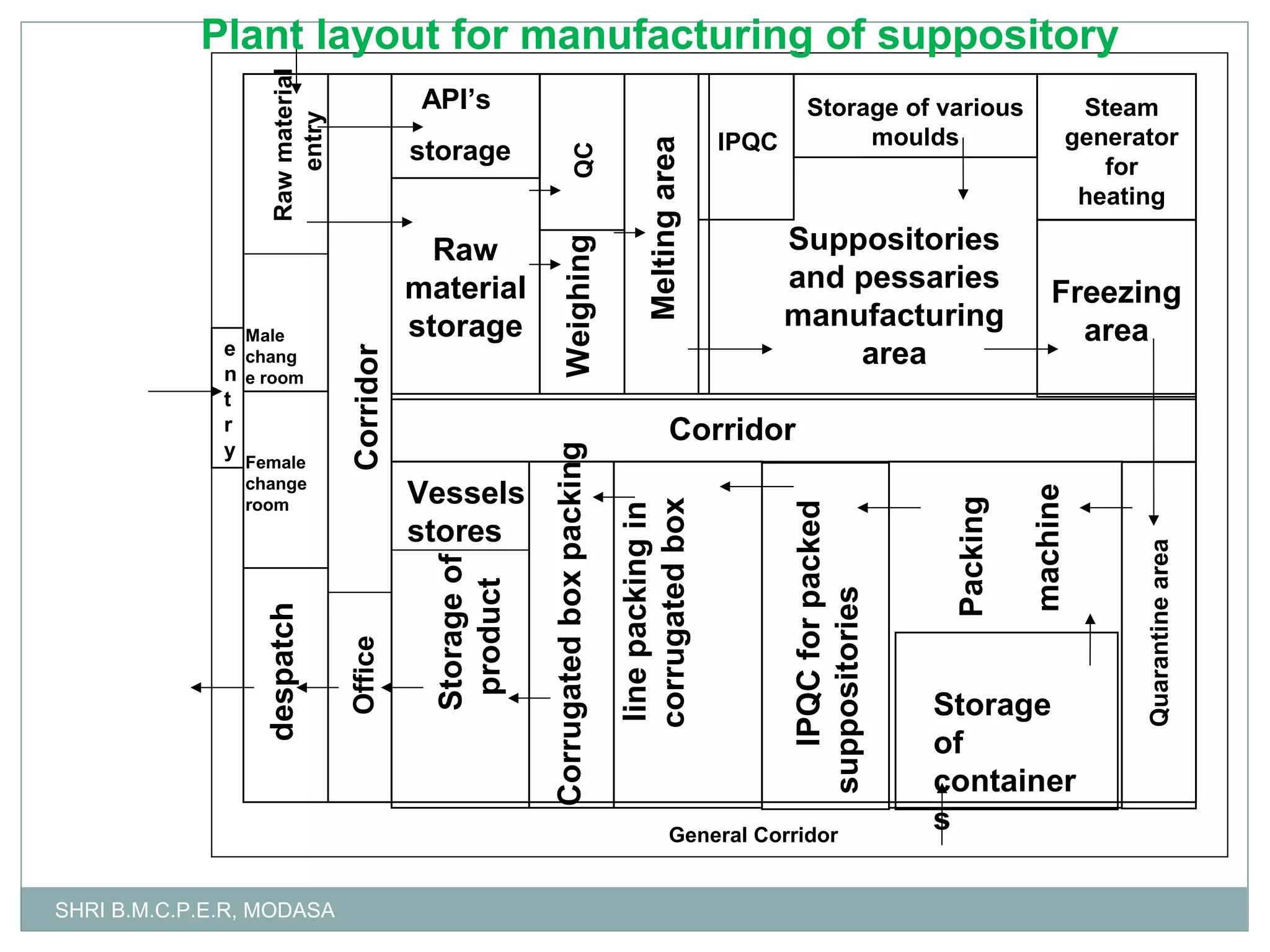
This document presents information on qualitative and quantitative plant layouts for semisolid dosage form manufacturing. It includes classifications of semisolid dosage forms, advantages, and requirements for external preparation and suppository plants based on Schedule M guidelines. Plant layout diagrams show designated areas for raw materials, manufacturing, filling, packaging, storage, and quality control. Specific requirements for topical preparation plants include manufacturing in an airlocked area with filtered air, exhaust systems, and temperature control.