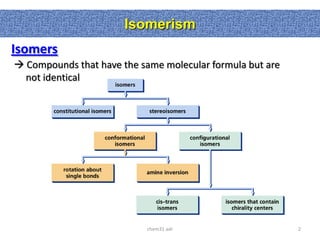
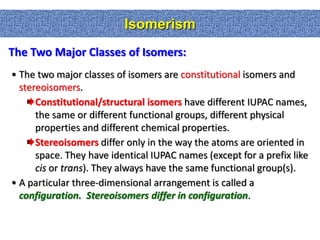
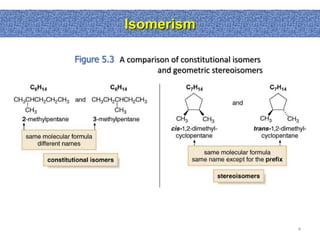
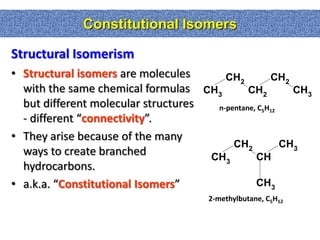
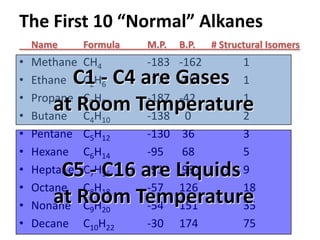
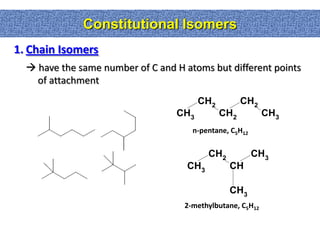
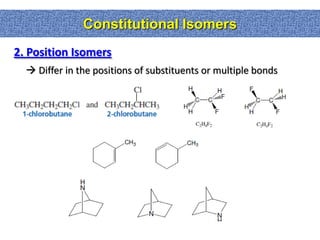
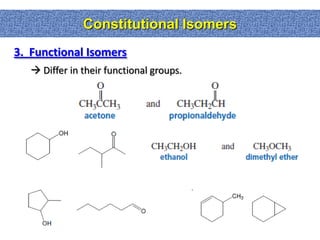
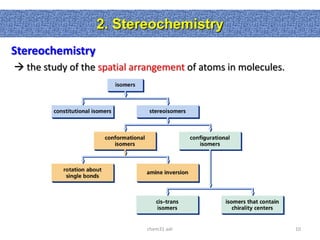
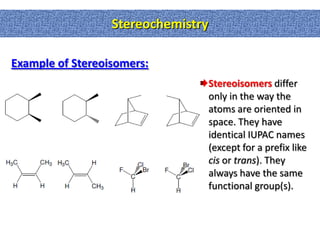
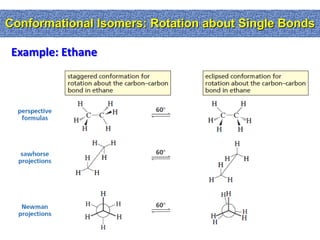
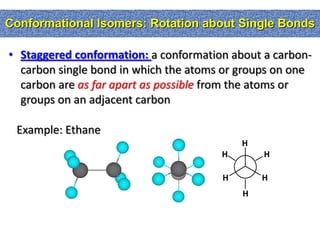
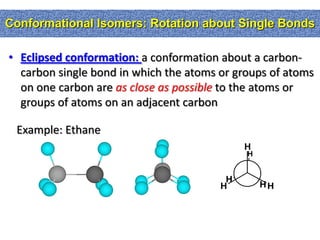
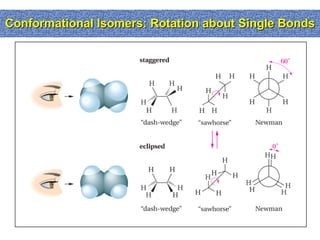
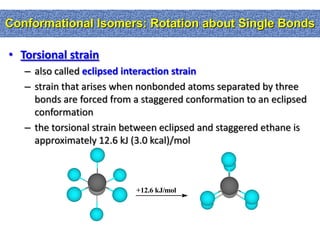
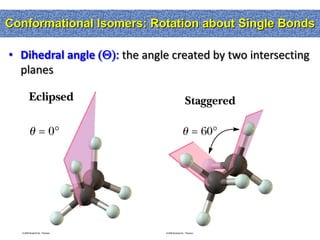
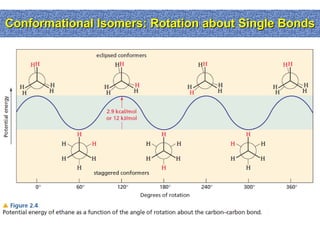
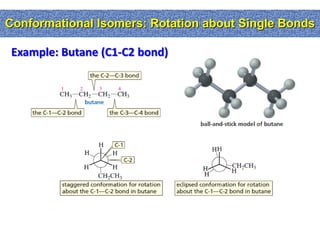
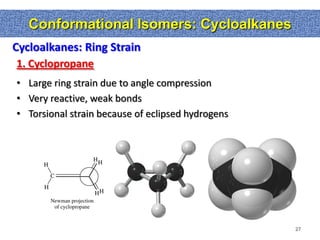
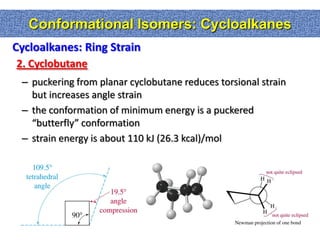
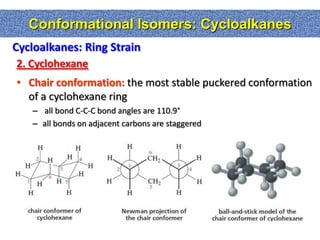
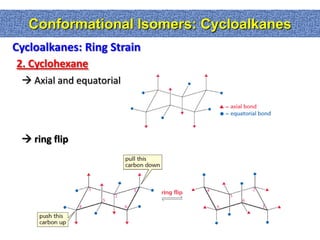
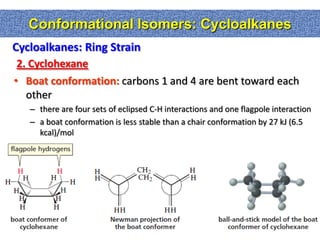
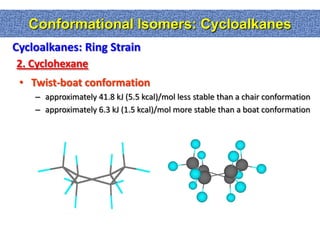
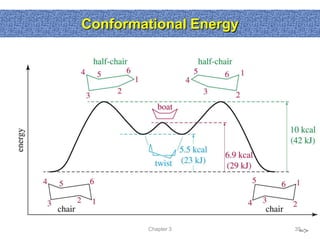
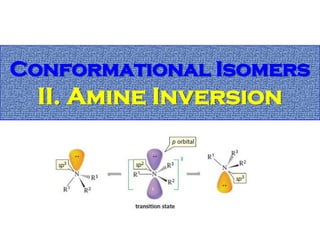
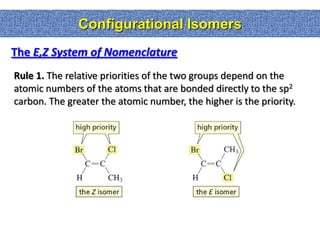
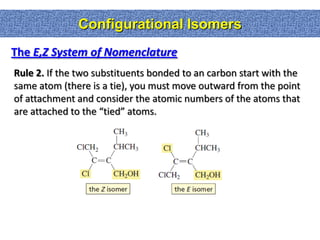
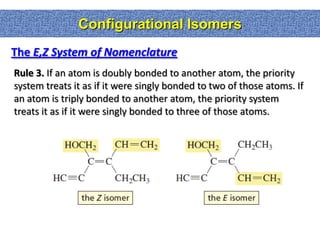
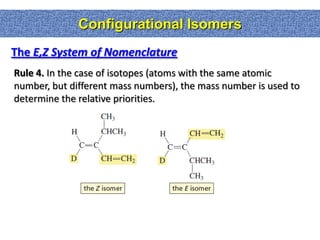
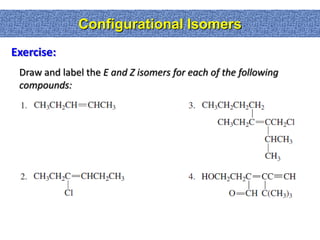
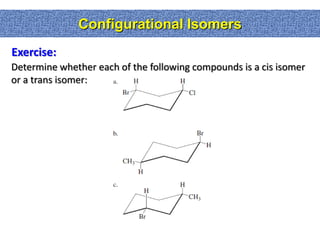
1. The document discussed different types of isomers including constitutional isomers, which have the same molecular formula but different connectivity, and stereoisomers, which have the same connectivity but different spatial arrangements.
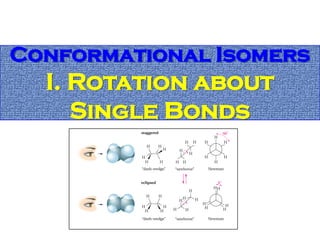
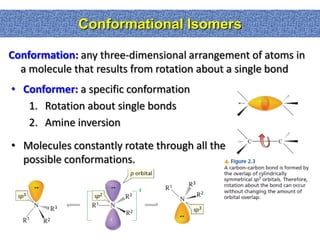
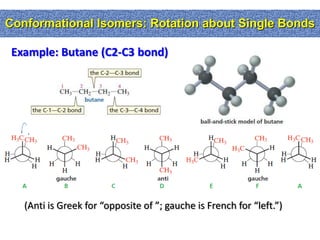
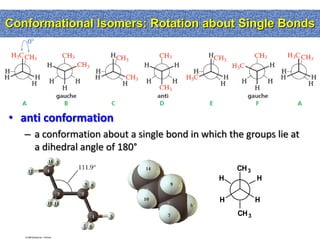
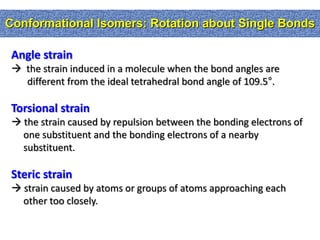
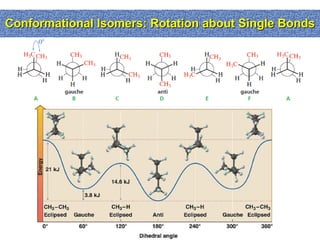
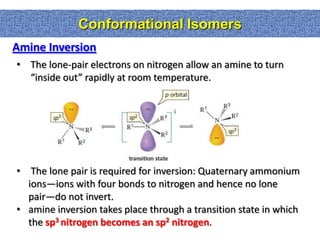
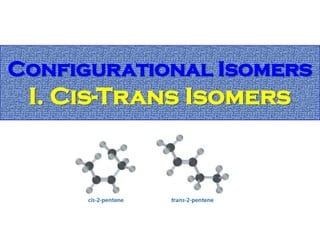
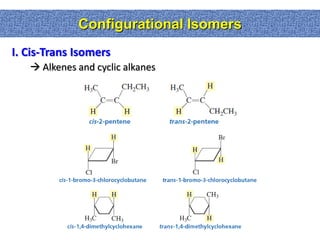
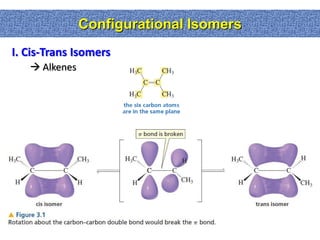
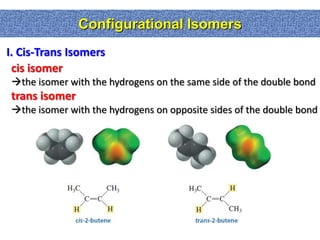
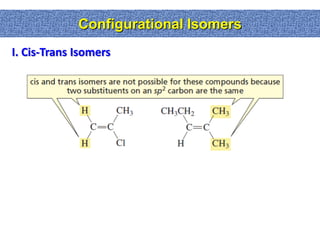
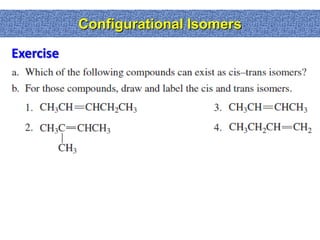
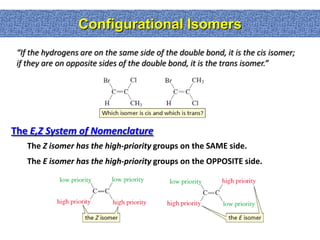
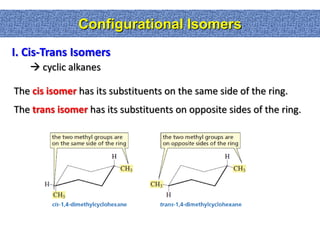
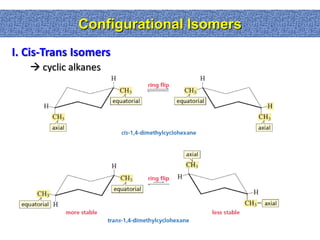
2. Conformational isomers result from rotation about single bonds, while configurational isomers include cis-trans isomers of alkenes and cyclic alkanes.
3. Isomerism, including constitutional, stereoisomers, conformational isomers, and configurational isomers, arises from different