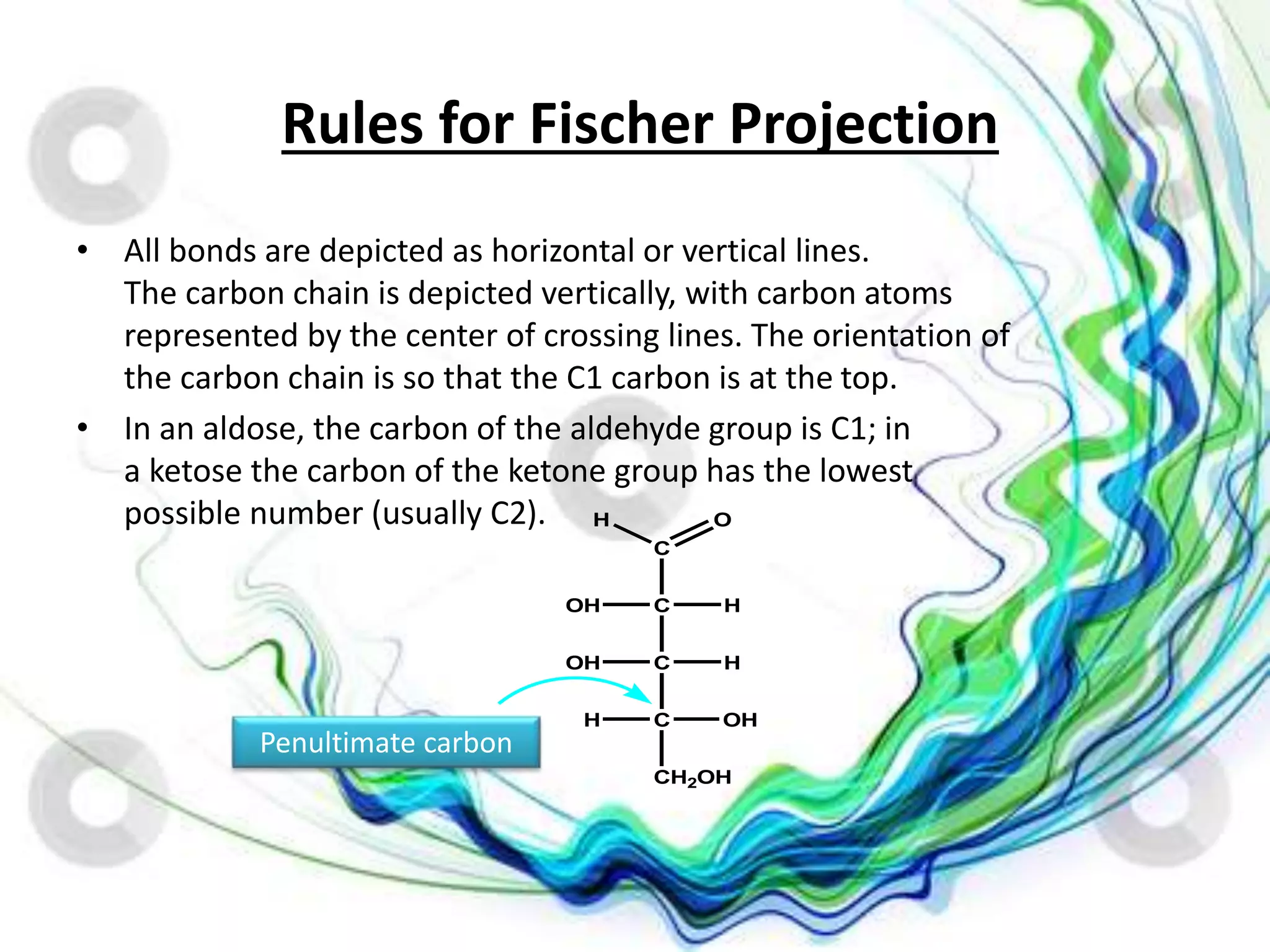
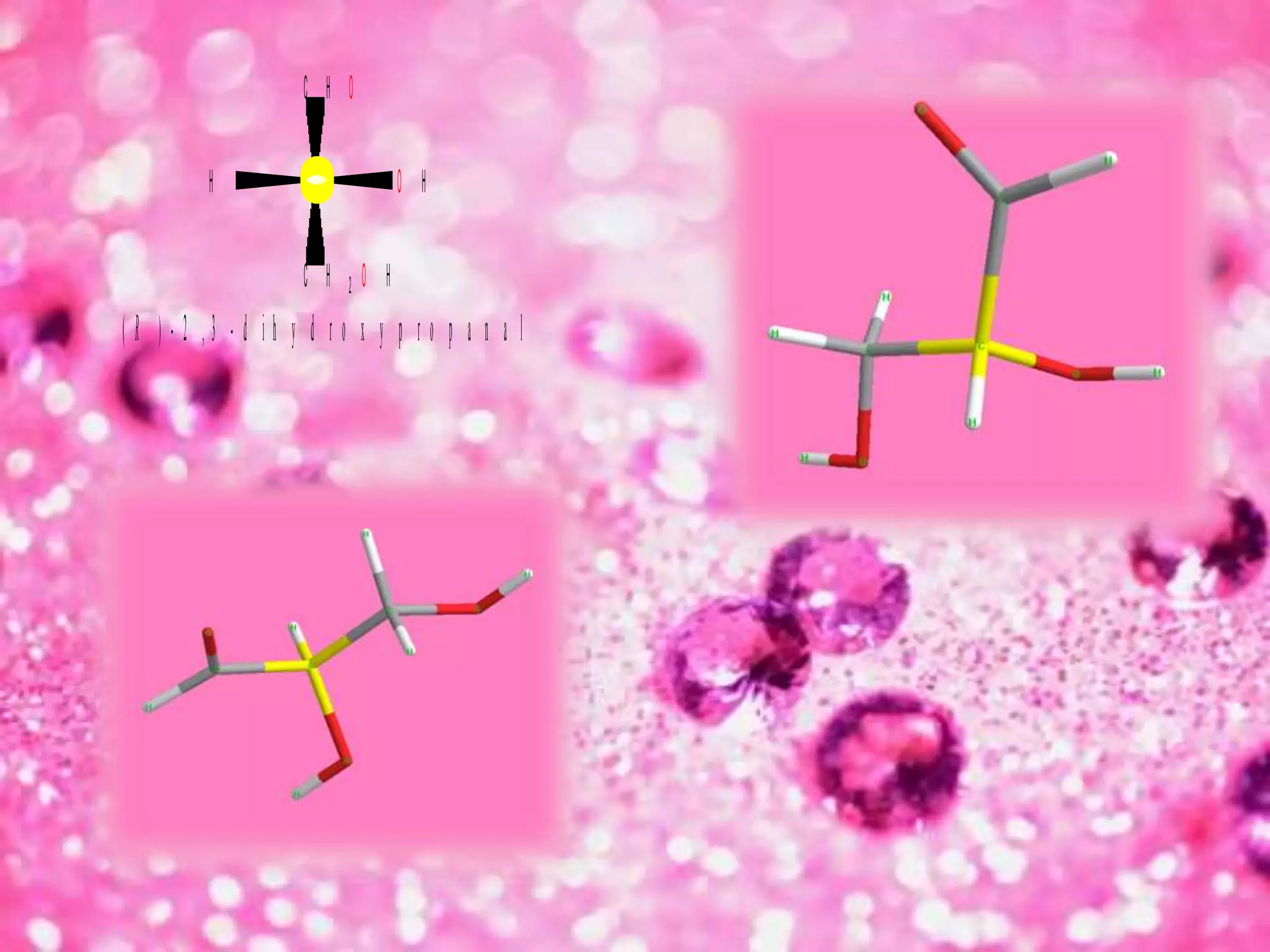
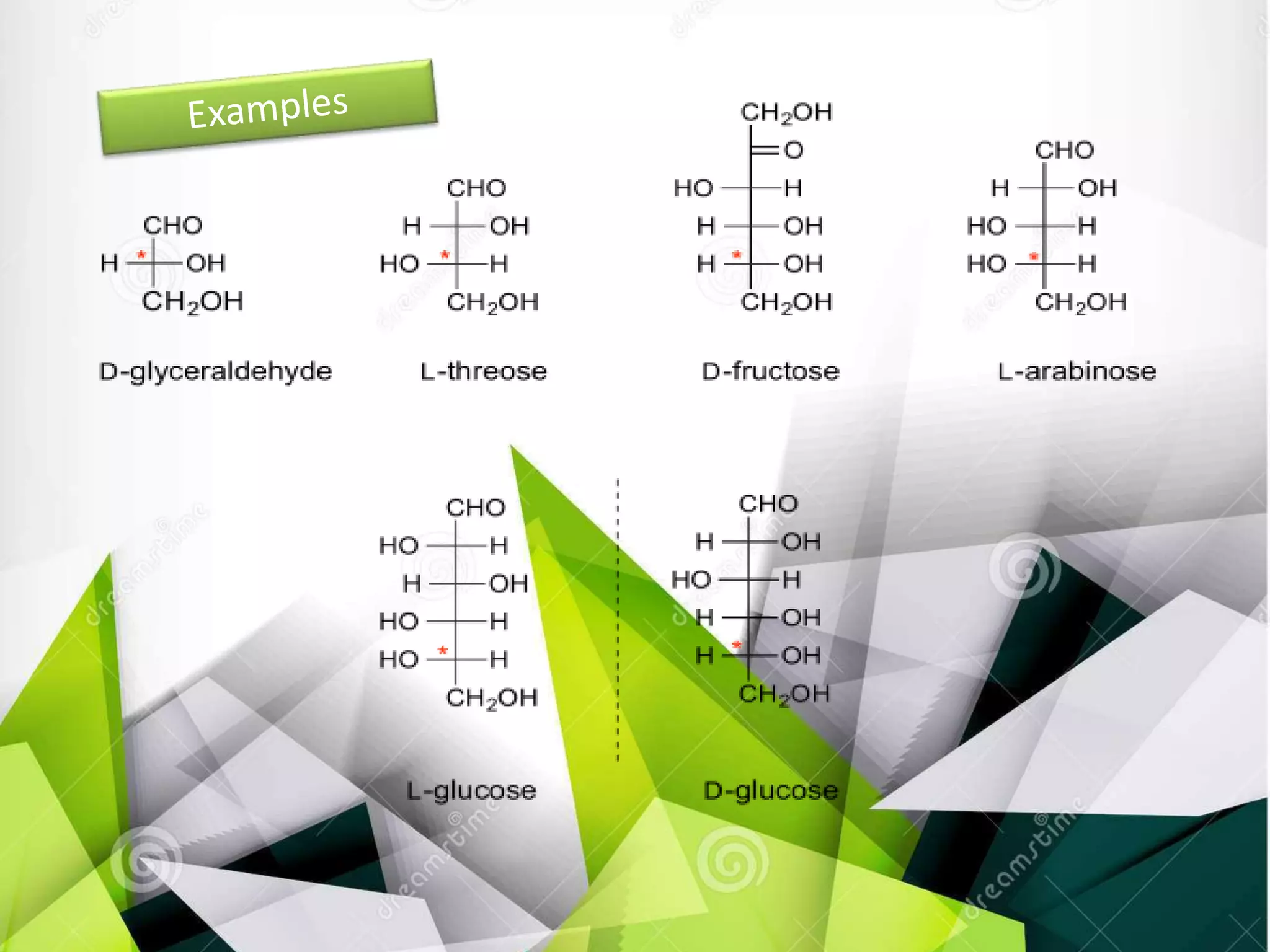
The document discusses the D and L nomenclature system used to designate the configurations of chiral carbons in carbohydrates and amino acids. It explains that D and L refer to the stereochemistry of glyceraldehyde, with D having the hydroxyl group on the right and L having it on the left in the Fischer projection. The rules for assigning D and L based on the orientation of functional groups around the penultimate carbon are described. In contrast to R/S systems, D and L name the configuration of the entire molecule based on a single stereocenter.