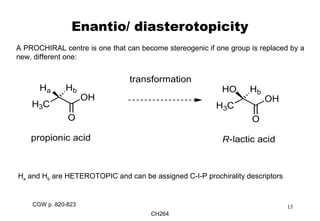
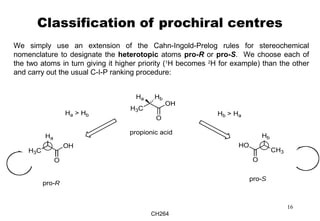
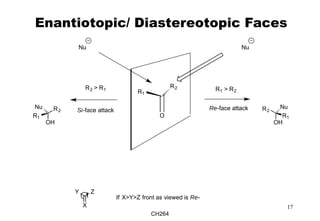
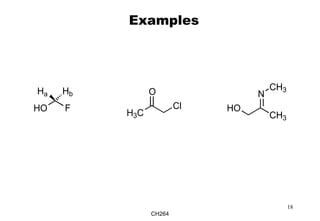
The document outlines the principles of stereochemistry in organic chemistry, focusing on Cahn-Ingold-Prelog rules for stereochemical assignments, differentiation between enantiomers and diastereomers, and the concept of chiral molecules without stereogenic centers. It highlights mechanisms for assigning stereochemistry, the uniqueness of diastereomers, and the properties of meso compounds, along with prochiral and stereogenic centers. Key concepts such as optical activity, axial and helical chirality, and examples of chiral molecules are also discussed.


![CH264
10
meso-Compounds
If a molecule has any symmetry element e.g. internal plane of symmetry, σ or
centre of inversion, i, it is rendered optically inactive and is designated meso-.
centre of inversion
CO2HHO2C
OHHO
meso-tartaric acid
CO2HHO2C
HO OH
CO2HHO2C
HO OH
(–)-tartaric acid(+)-tartaric acid
HO2C
CO2H
OH
OH
HO2C
CO2H
OH
OH
R
R S
S
S
R
HO2C
CO2H
OH
OH
m.p. 206°m.p. 168-170°m.p. 168-170°
[α]D = +12° (water, 20°C) [α]D = –12° (water, 20°C) [α]D = 0° (water, 20°C)](https://image.slidesharecdn.com/ch264-1shapes-140621101809-phpapp02/85/Organic-Chemistry-Year-2-Mechanism-and-Stereochemistry-Lecture-1-10-320.jpg)