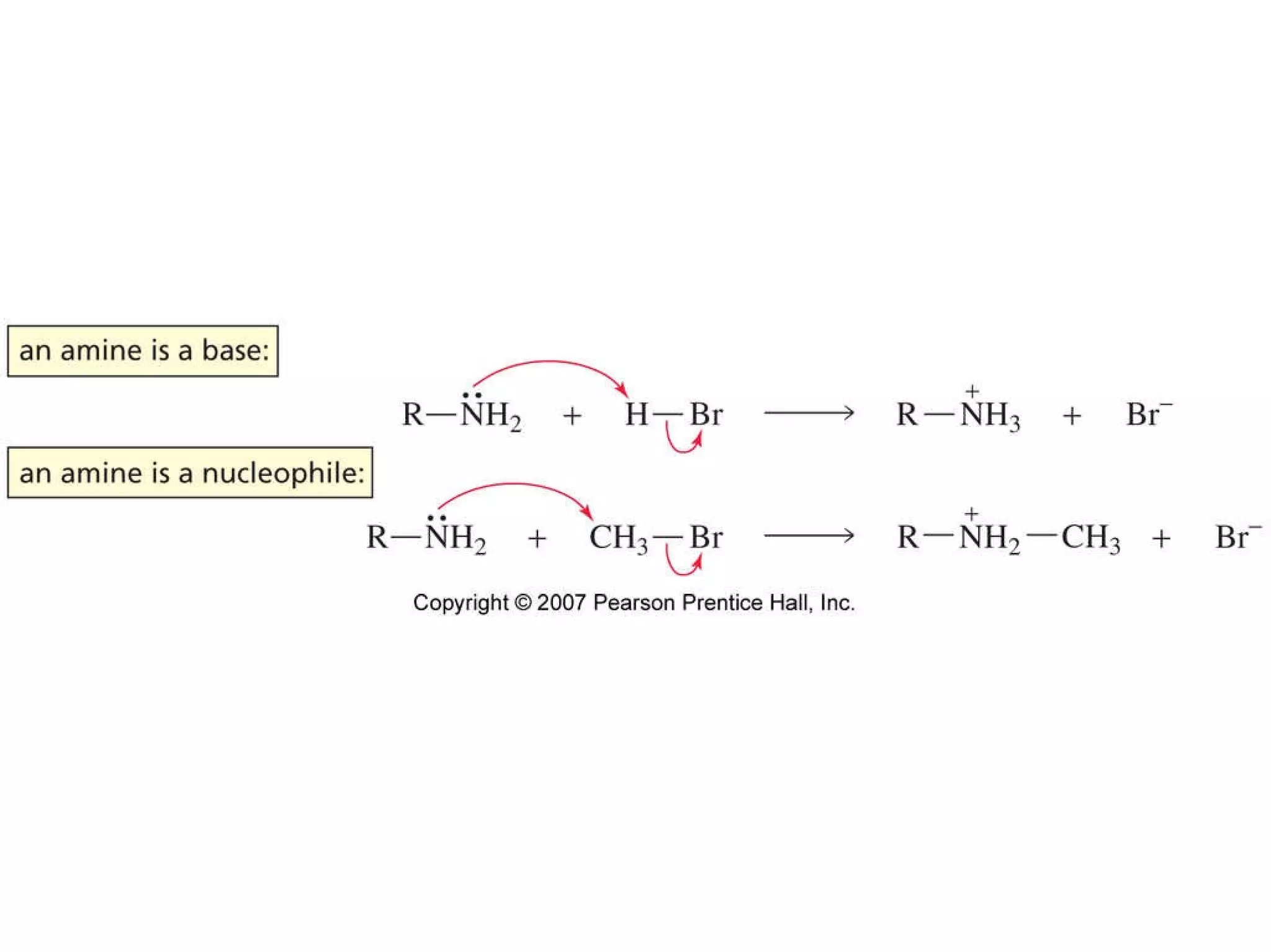
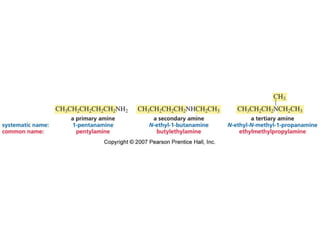
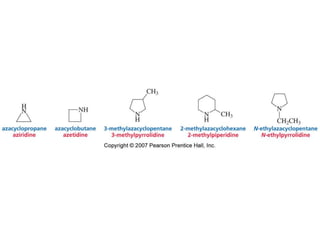
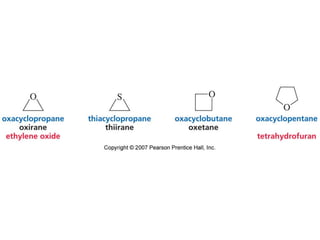
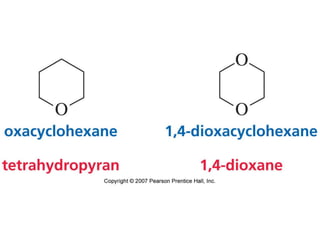
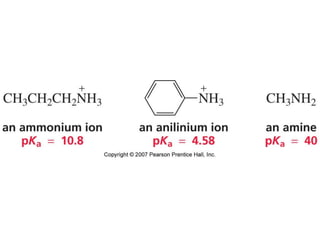
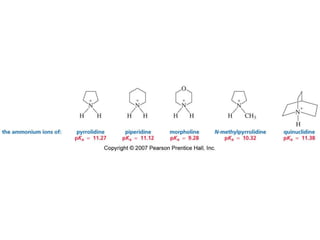
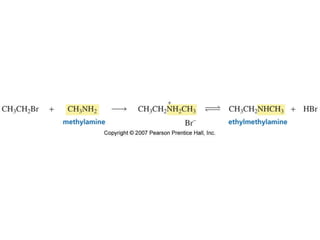
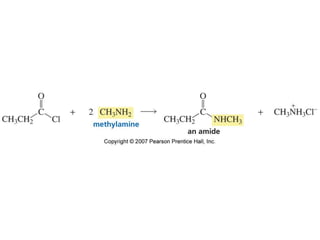
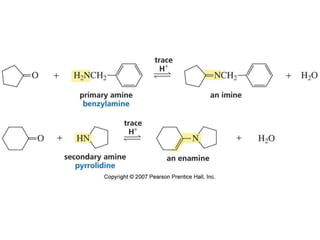
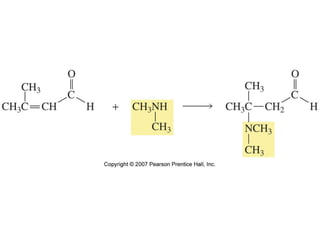
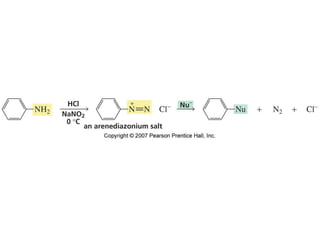
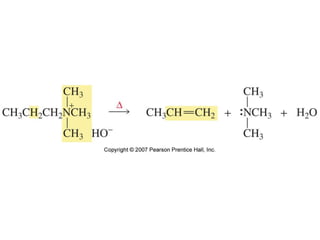
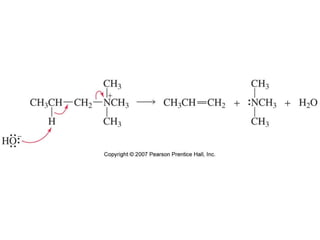
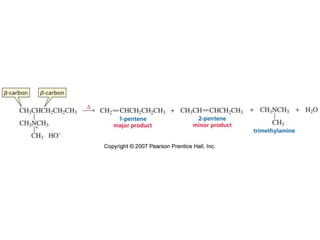
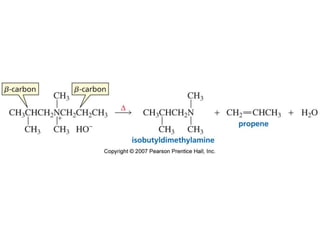
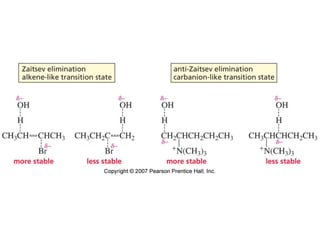
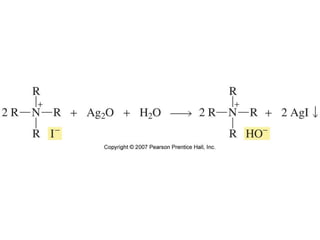
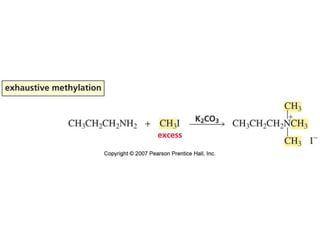
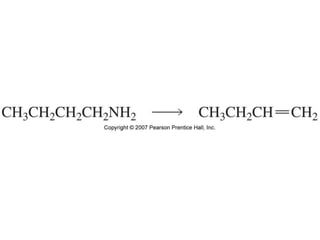
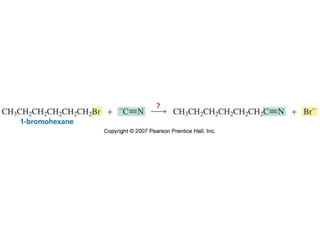
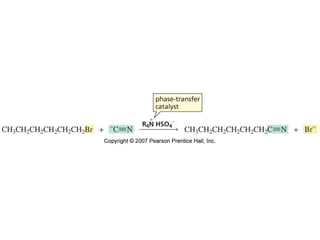
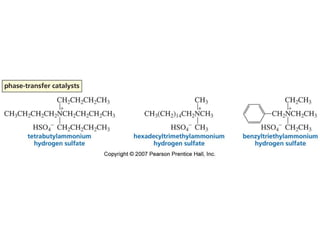
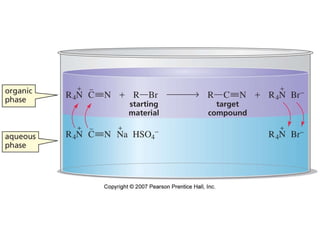
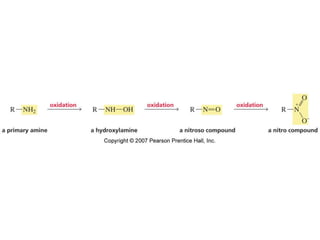
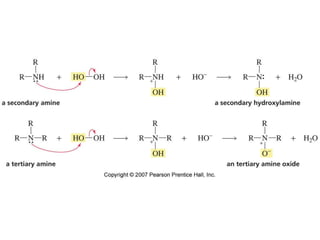
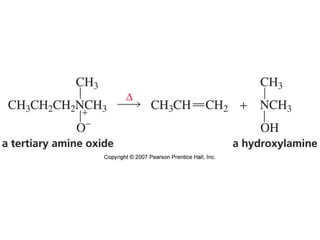
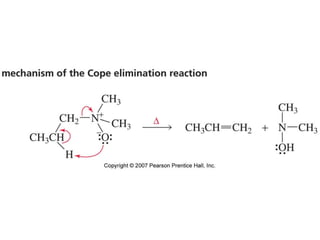
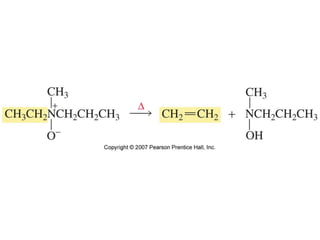
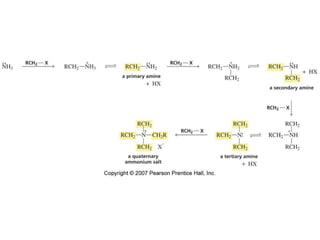
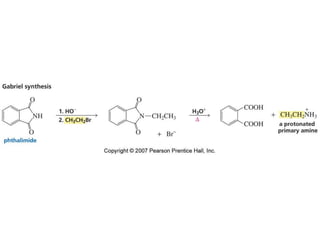
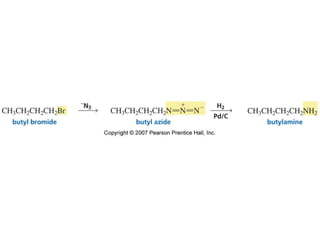
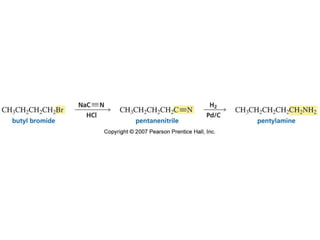
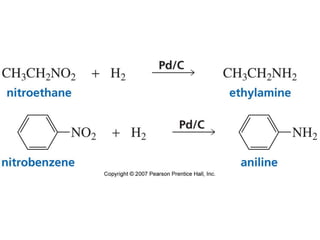
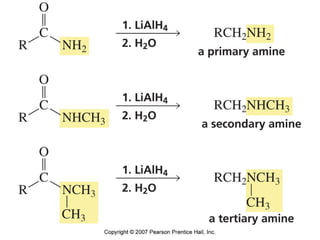
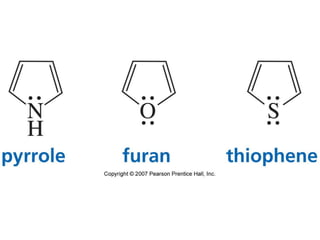
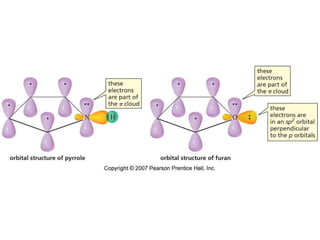
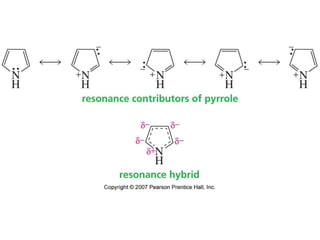
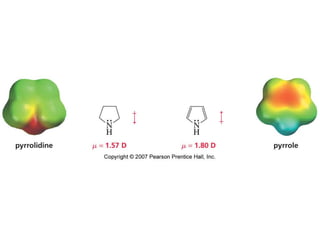
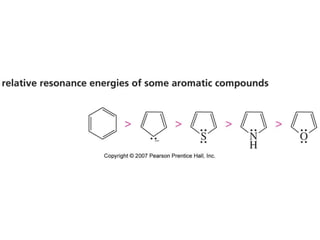
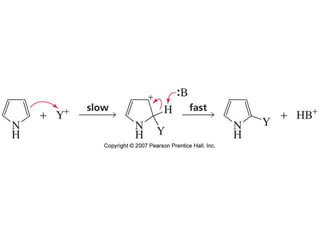
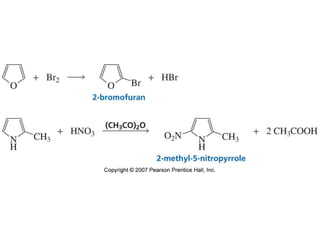
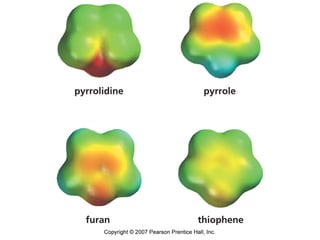
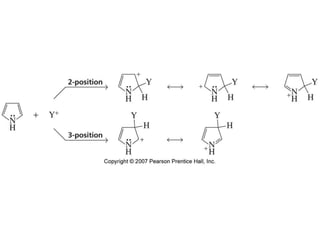
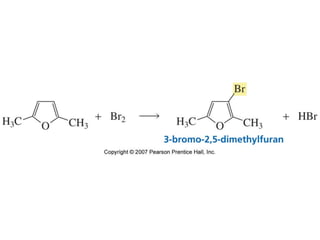
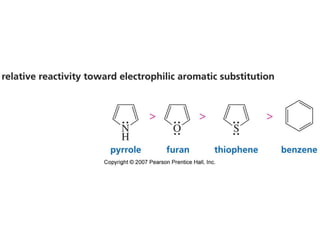
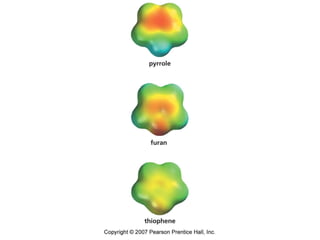
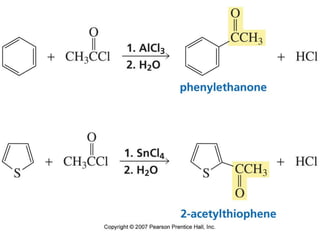
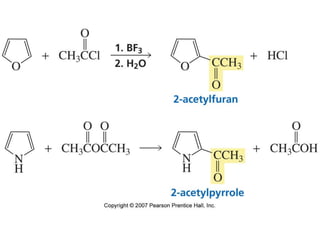
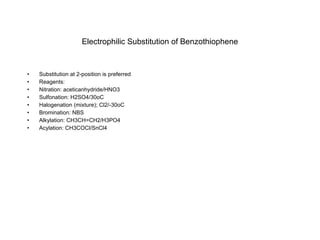
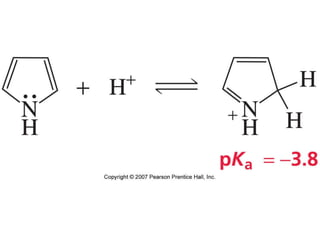
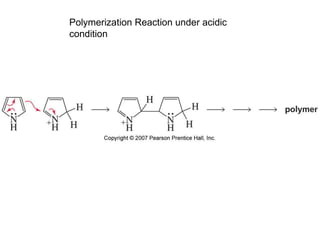
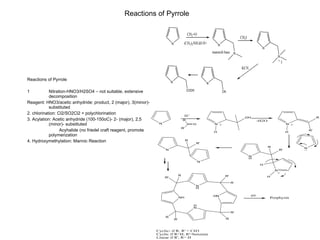
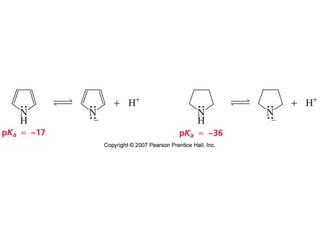
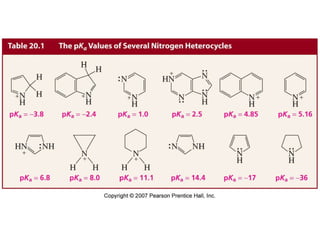
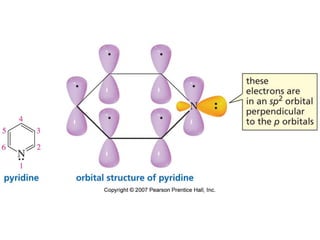
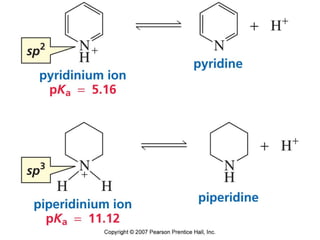
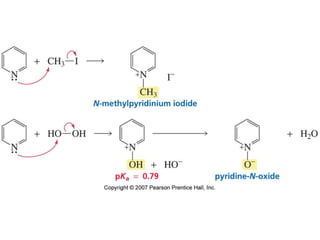
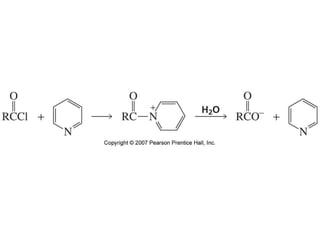
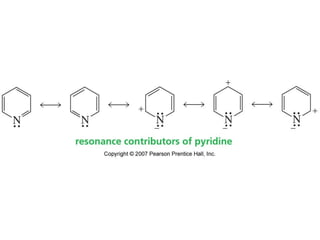
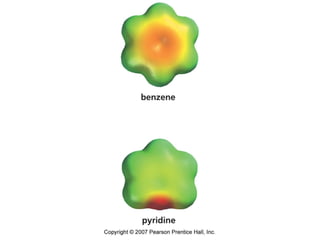
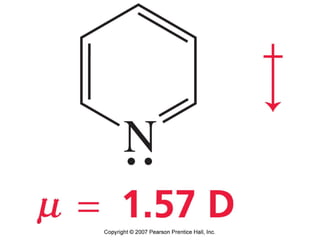
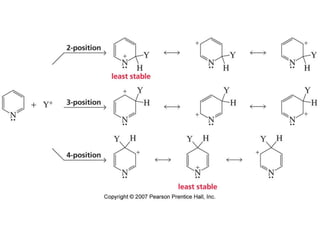
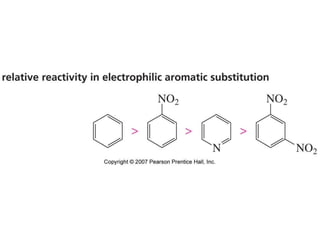
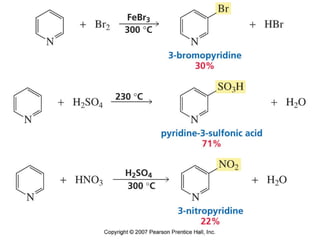
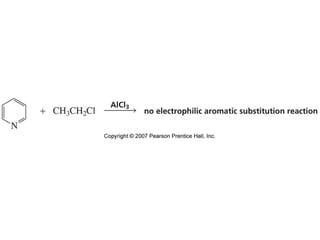
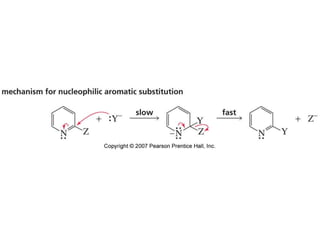
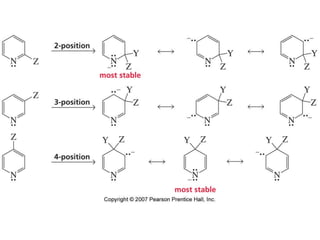
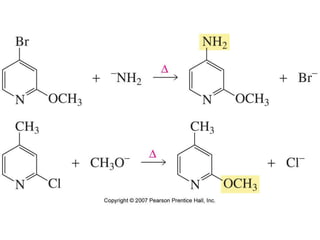
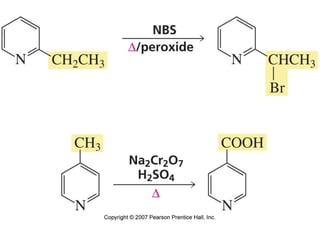
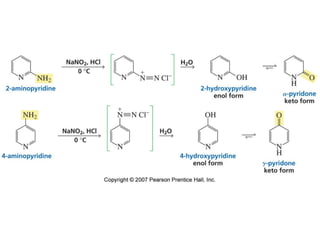
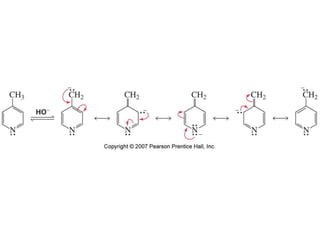
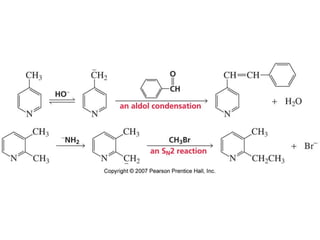
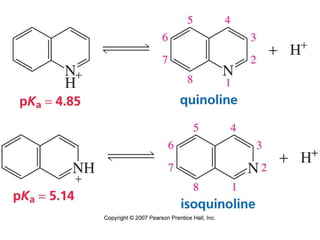
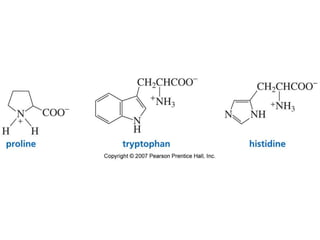
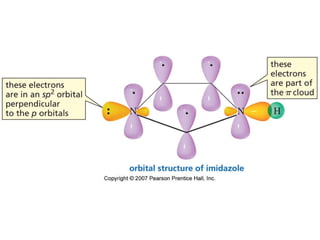
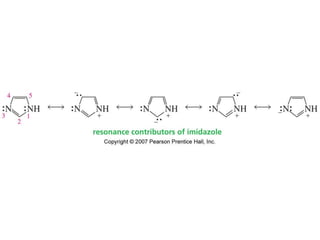
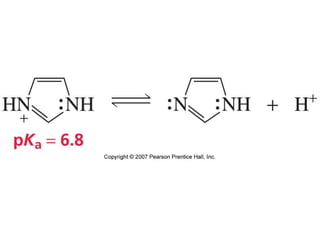
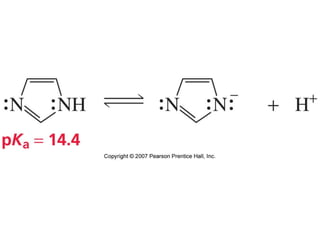
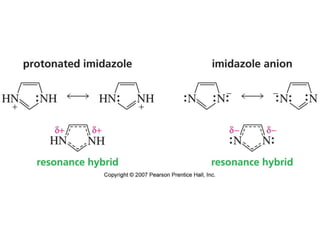
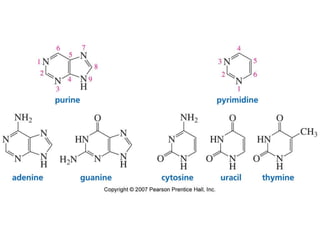
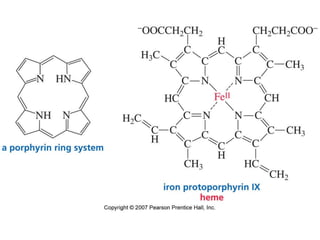
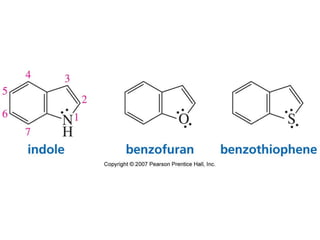
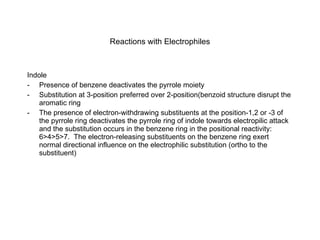
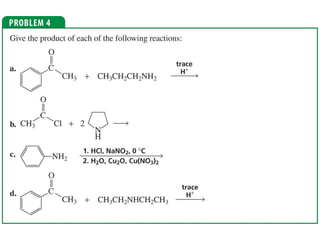
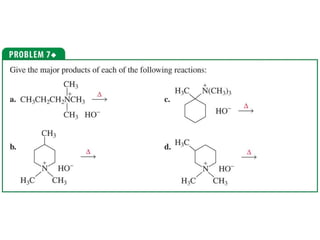
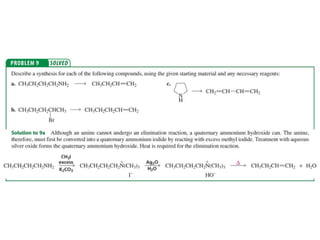
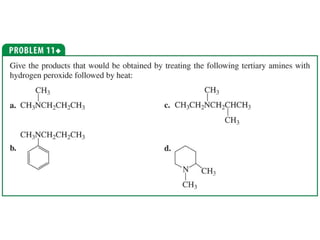
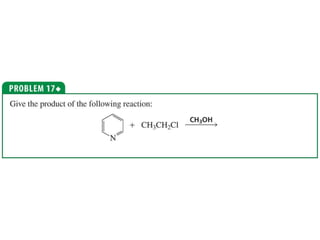
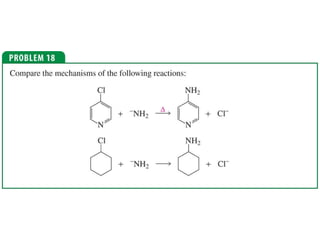
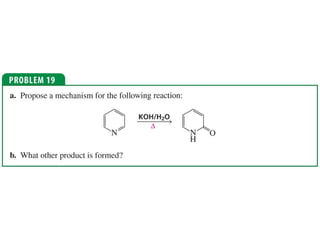
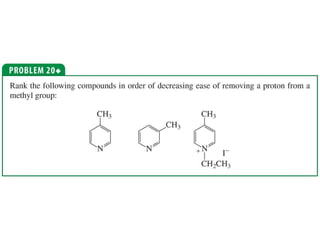
The document summarizes electrophilic substitution reactions of various aromatic heterocyclic compounds. For pyrrole, substitution prefers the 2-position and reagents include nitric acid, chlorine, and acetic anhydride. For furan, substitution occurs at the 2 and 5-positions. For benzothiophene, substitution prefers the 2-position with reagents like nitric acid and chlorine. Polymerization can occur under acidic conditions for pyrrole. Indole substitution prefers the 3-position and is deactivated by electron-withdrawing groups. For benzofuran, substitution occurs at the alpha position rather than beta due to oxygen's directing effect.