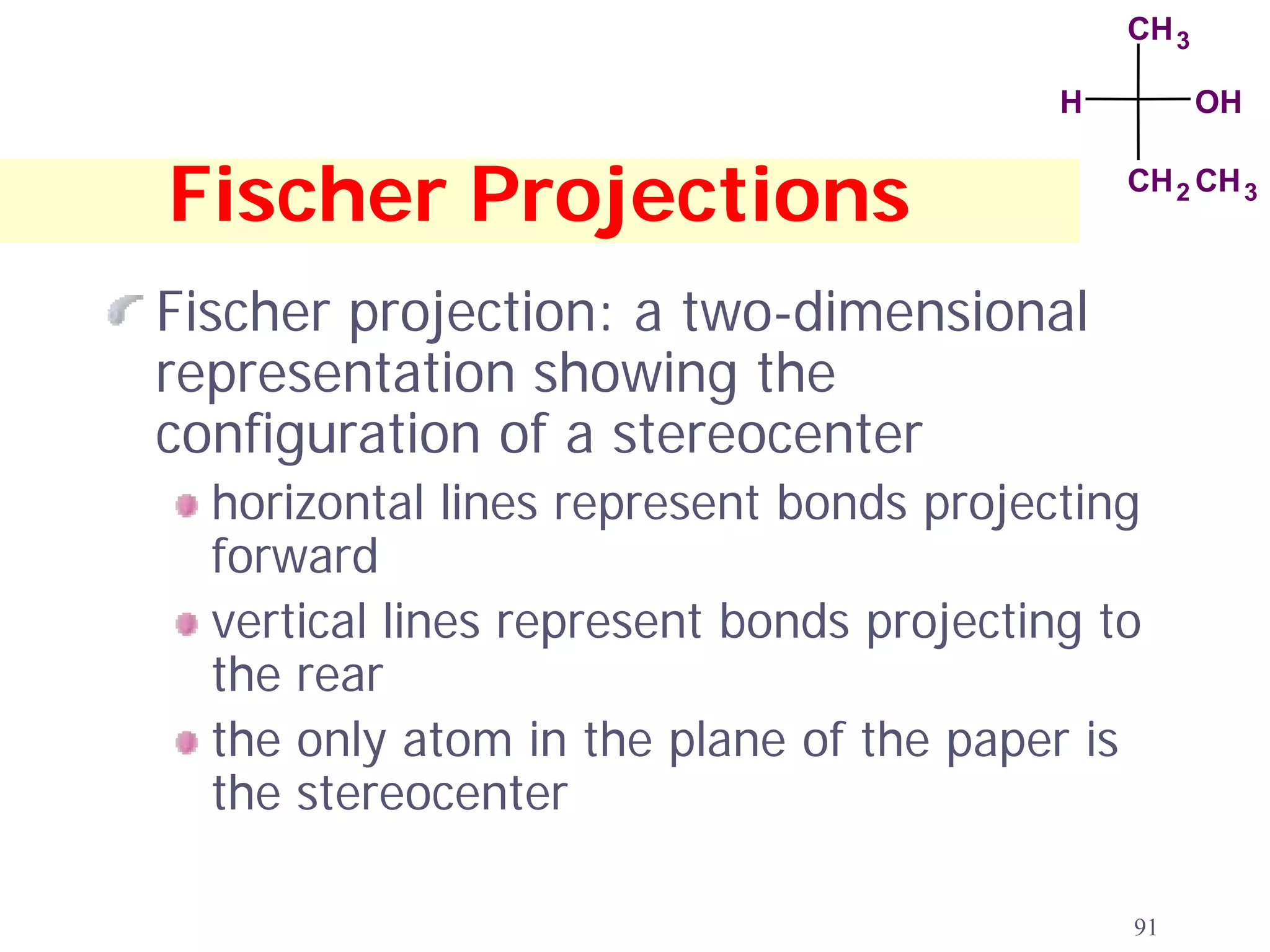
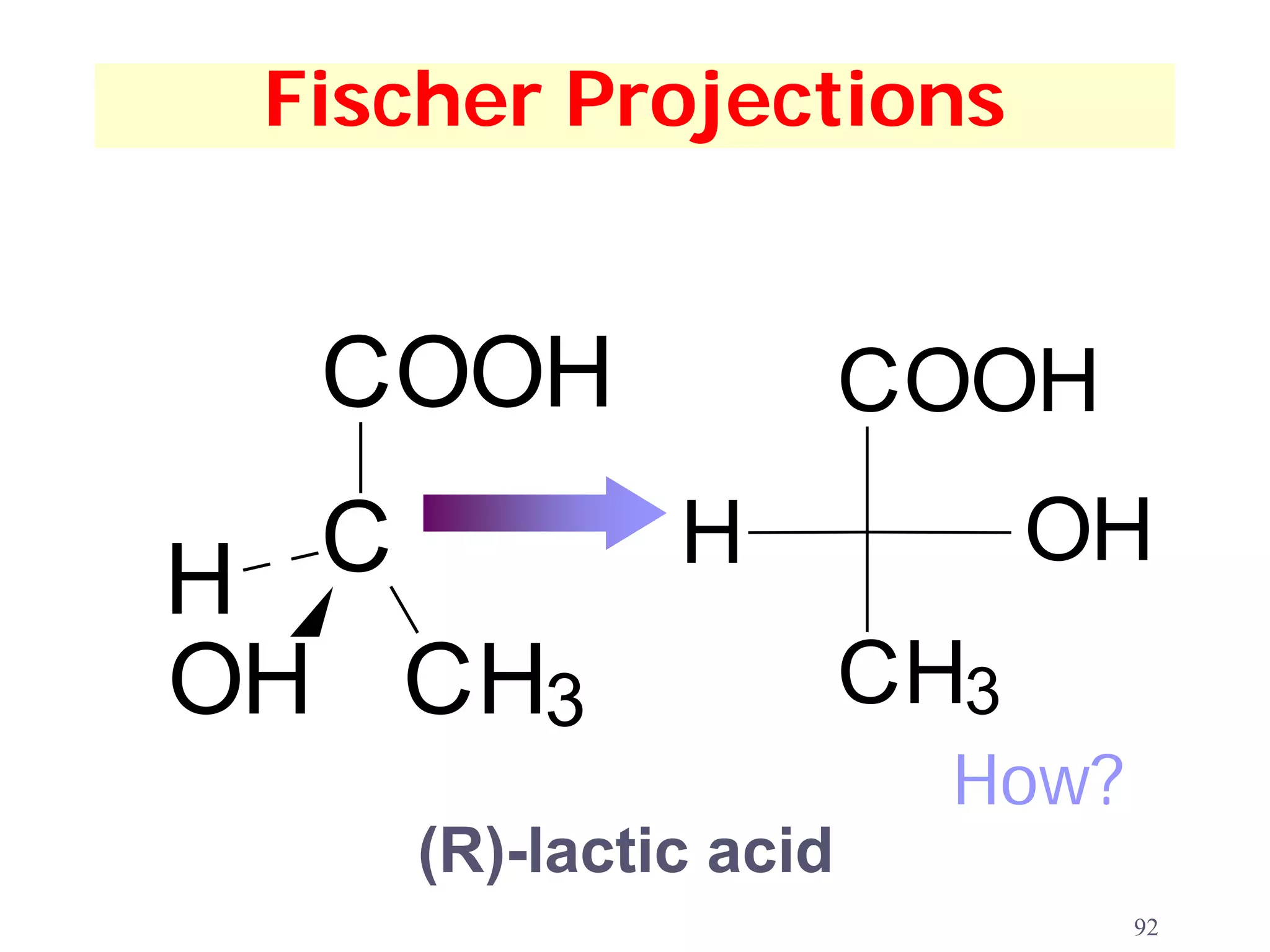
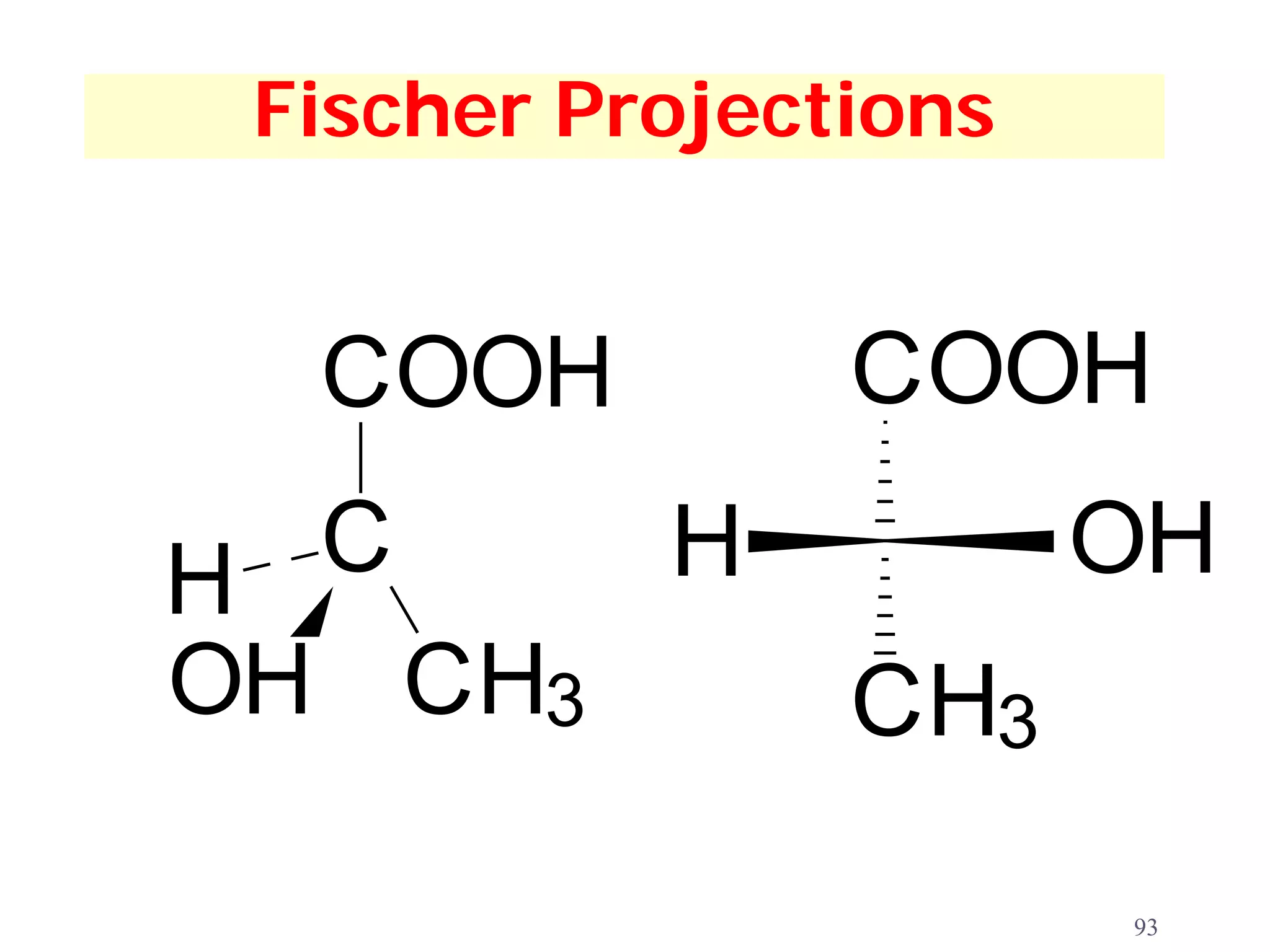
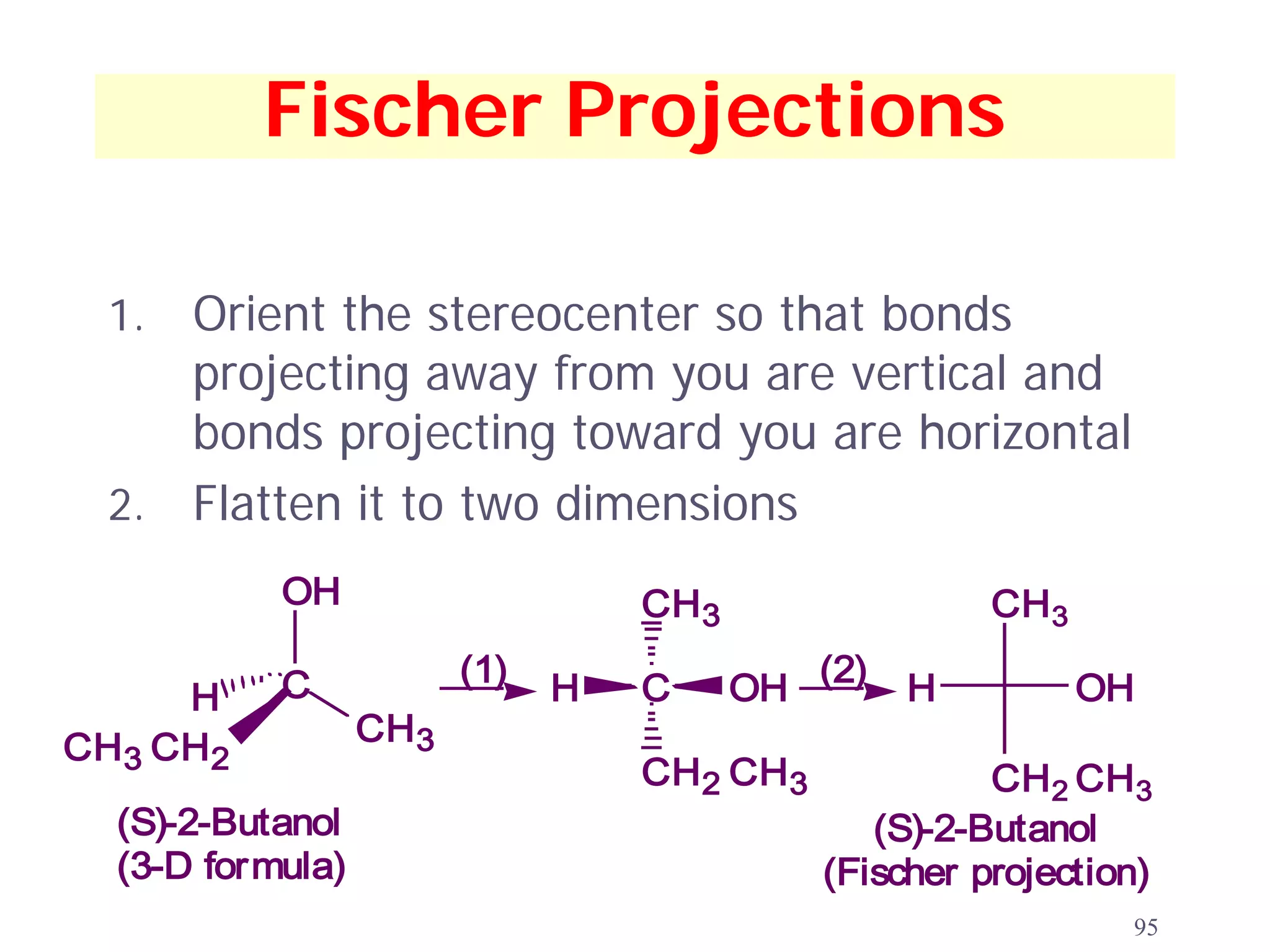
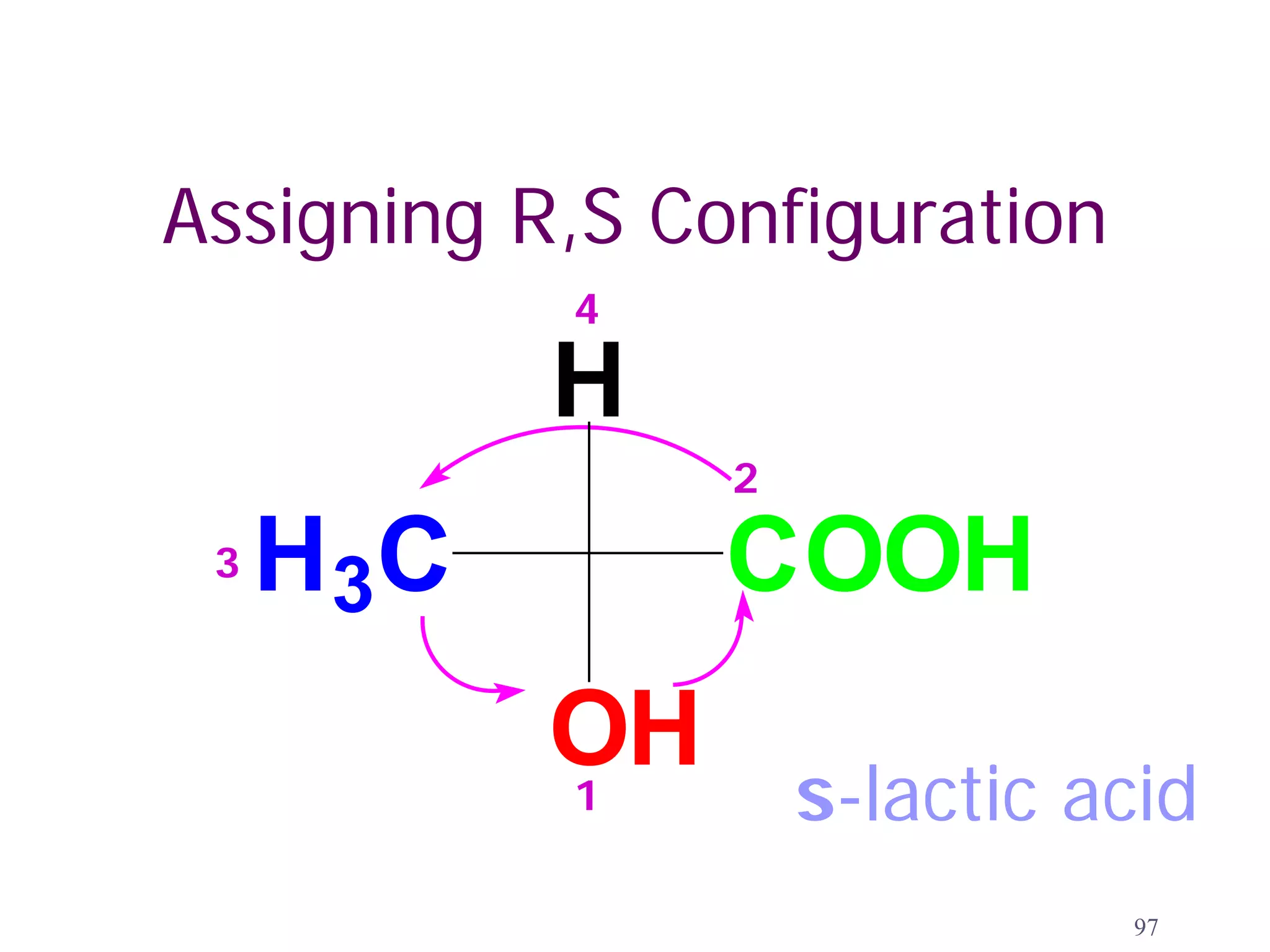
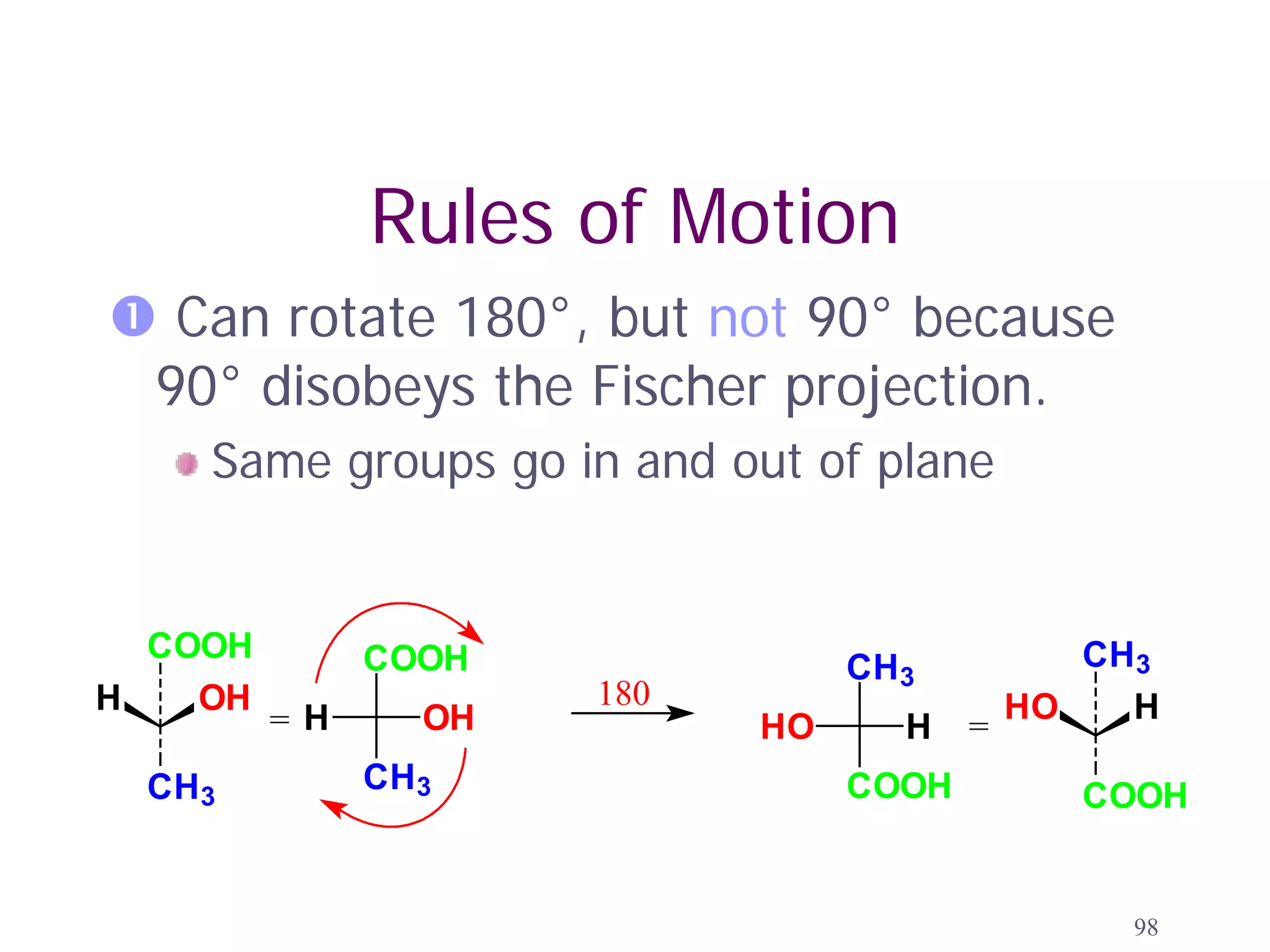
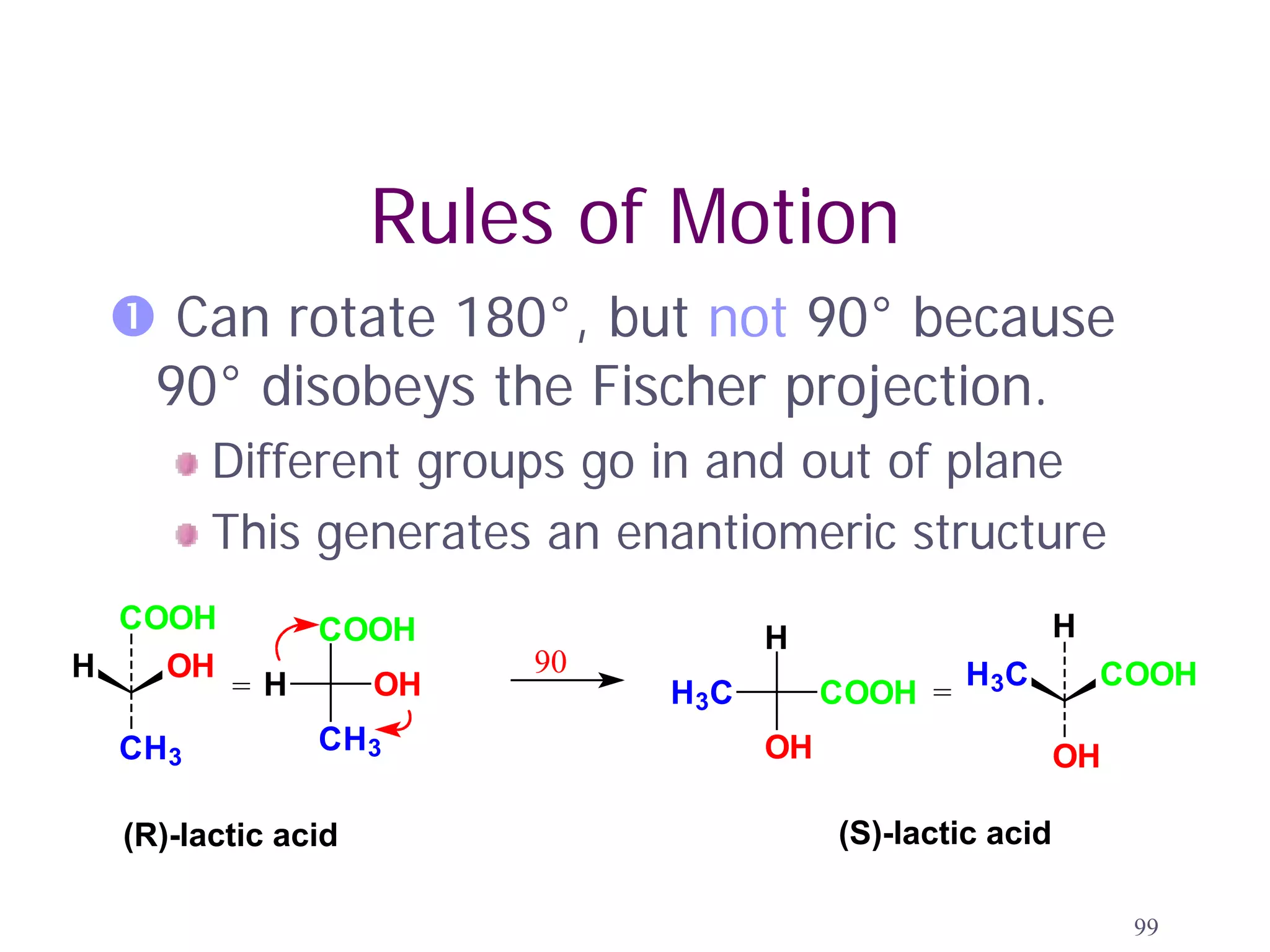
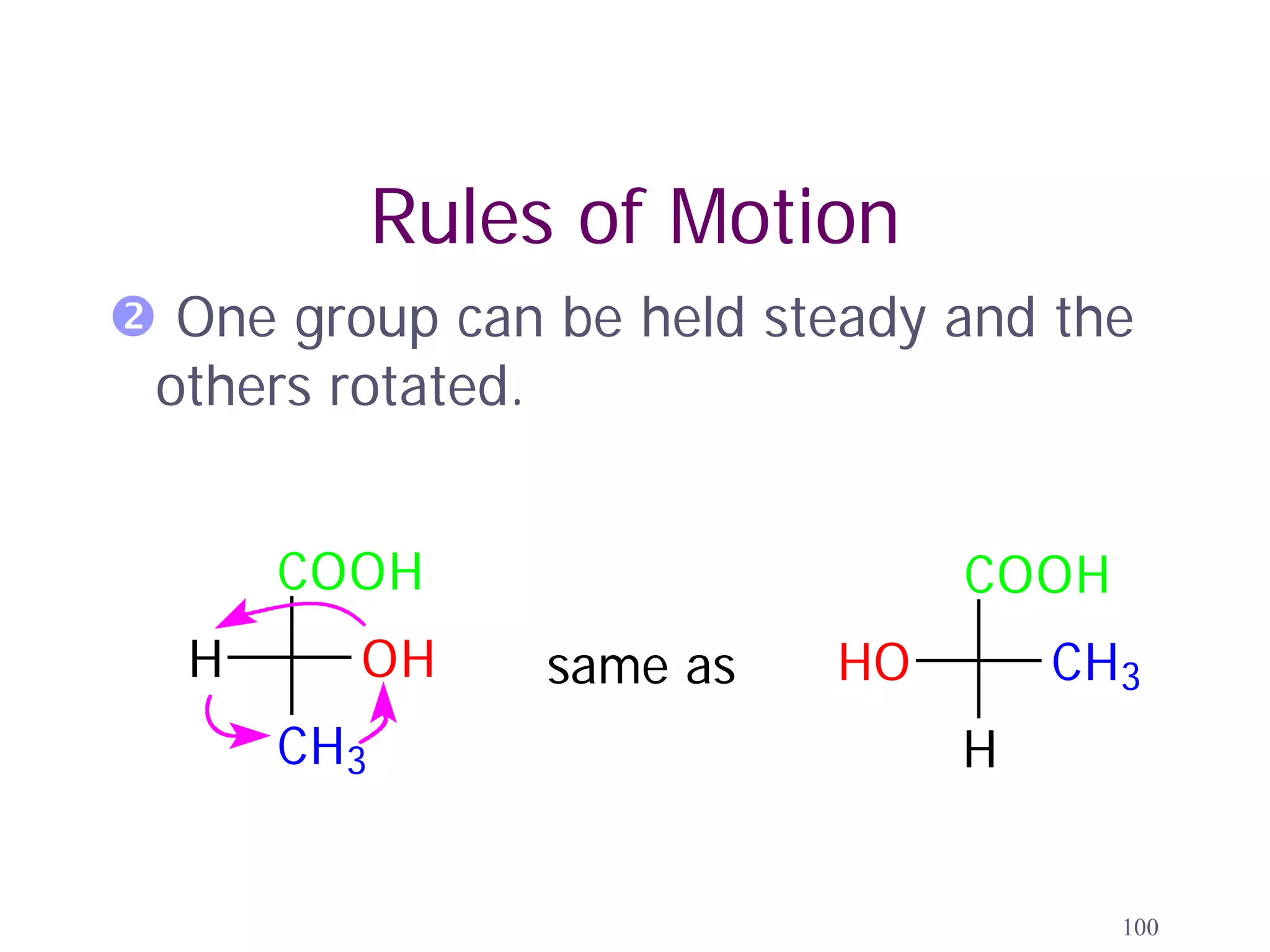
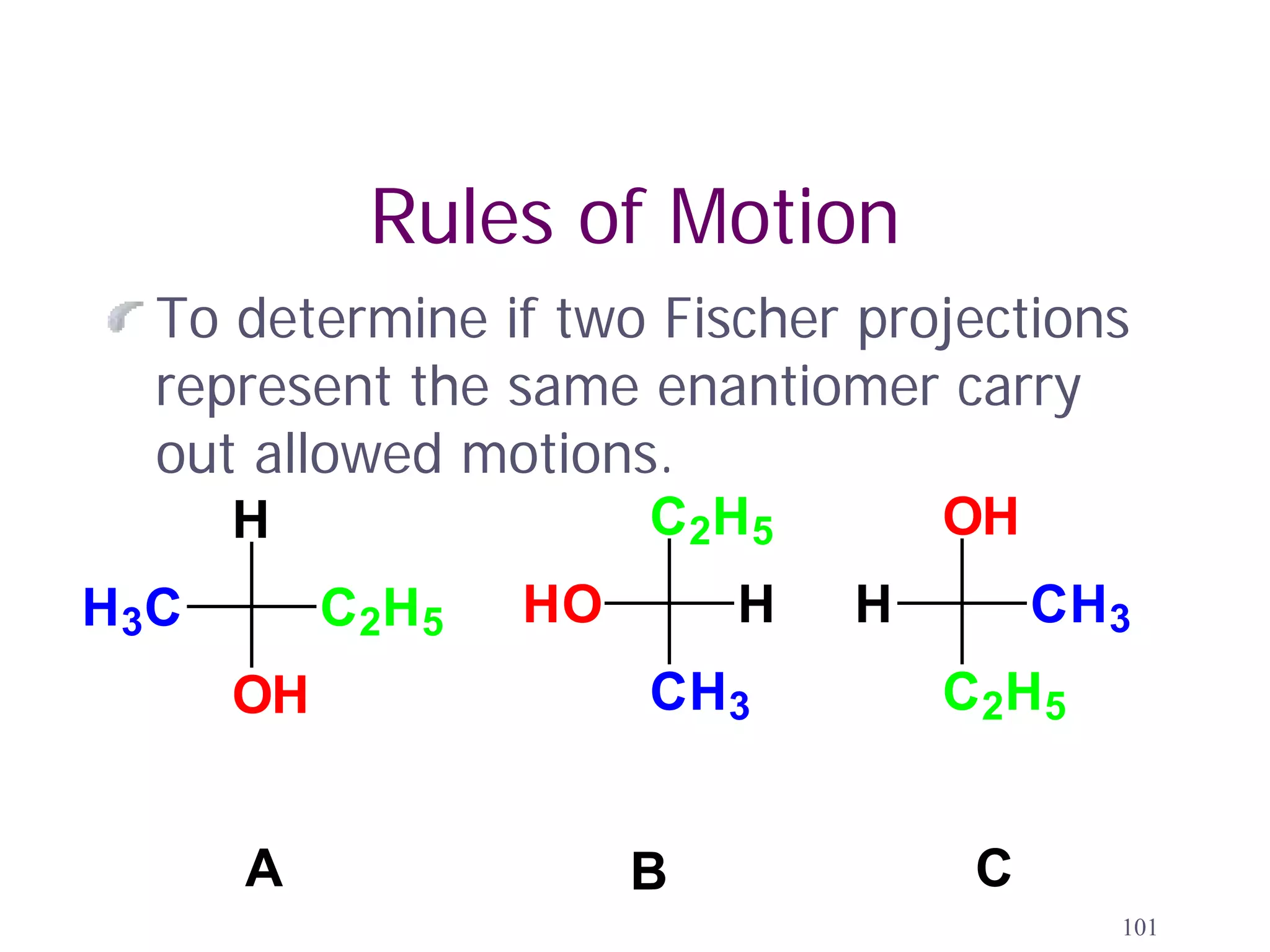
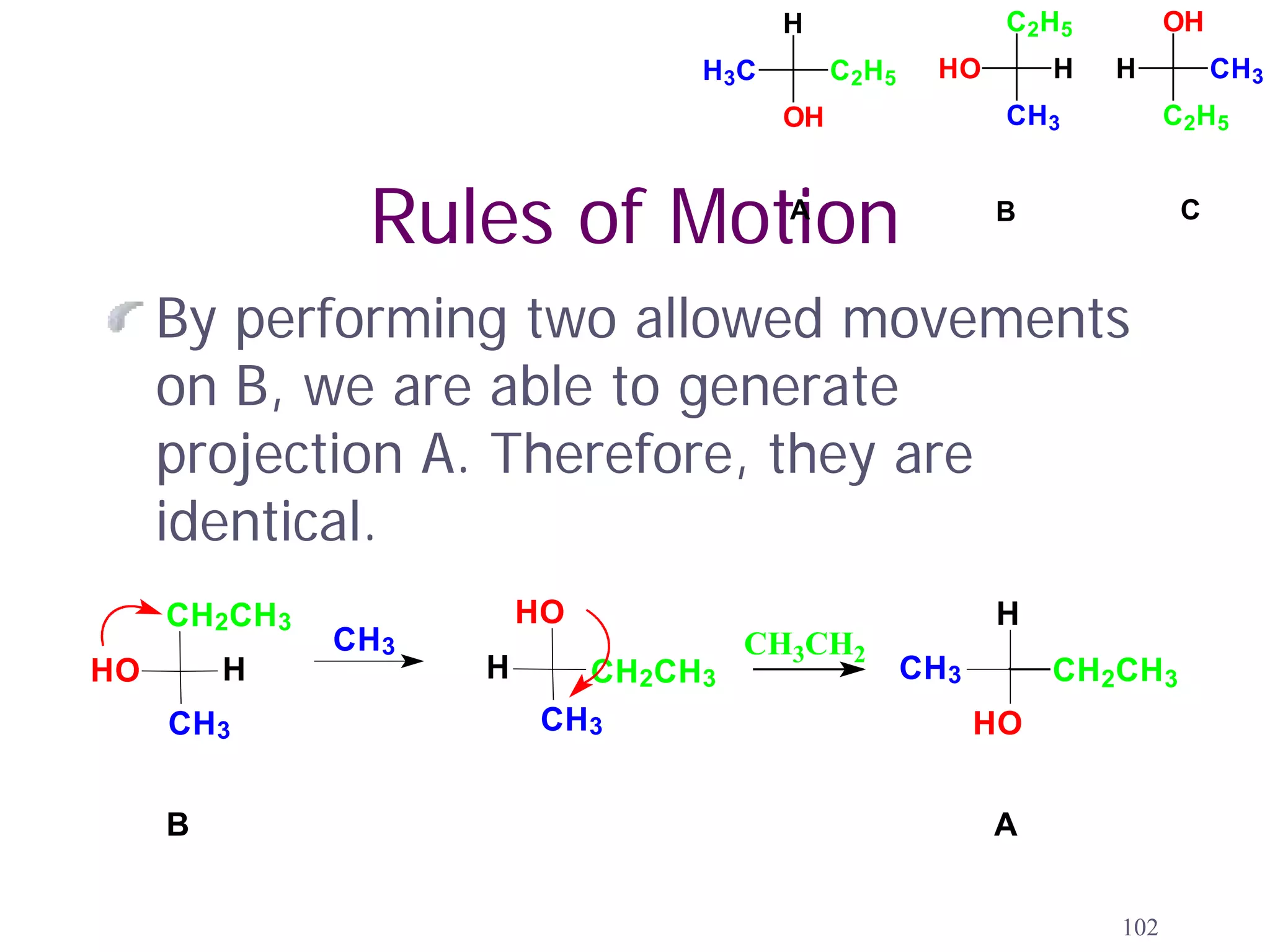
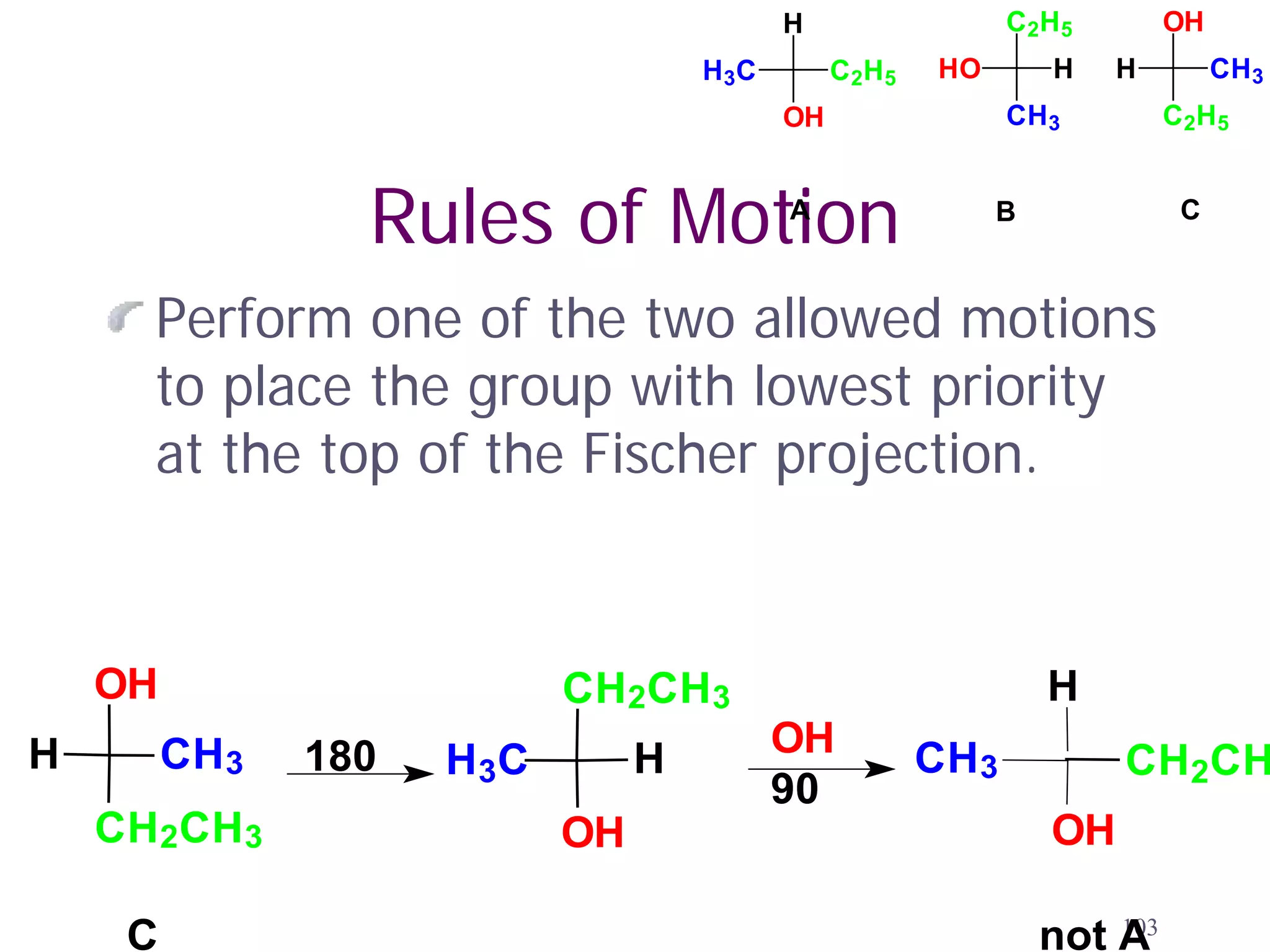
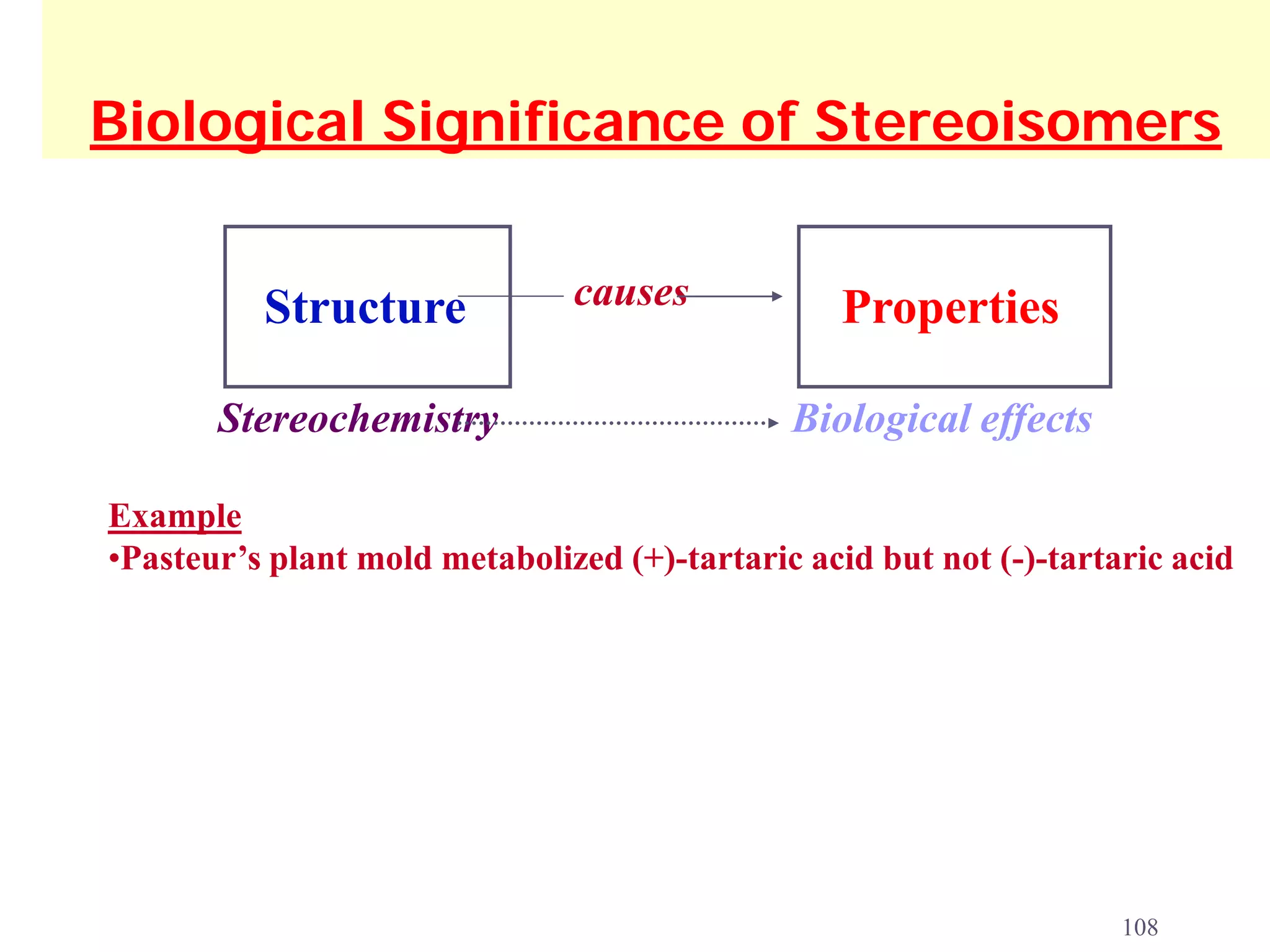
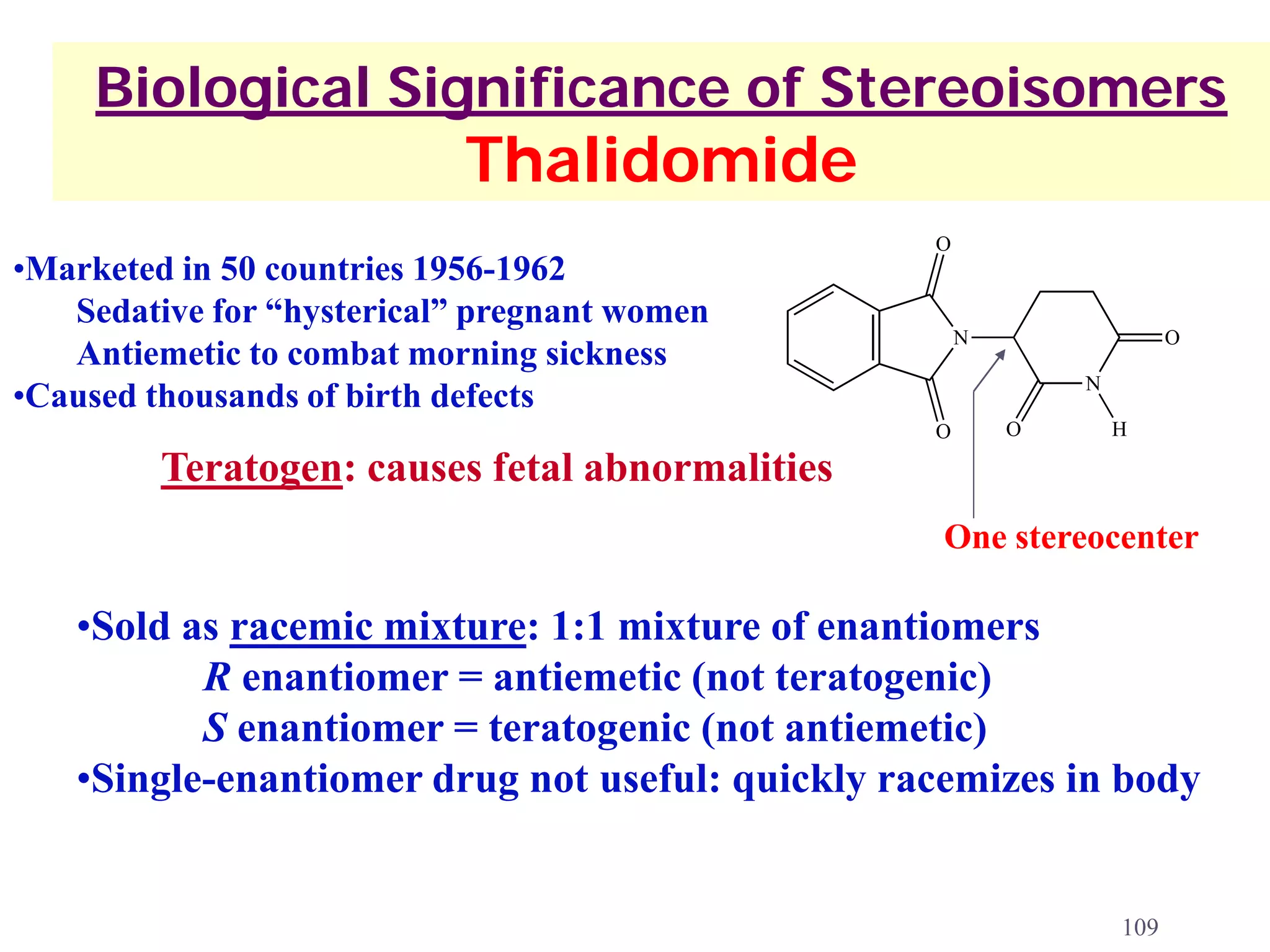
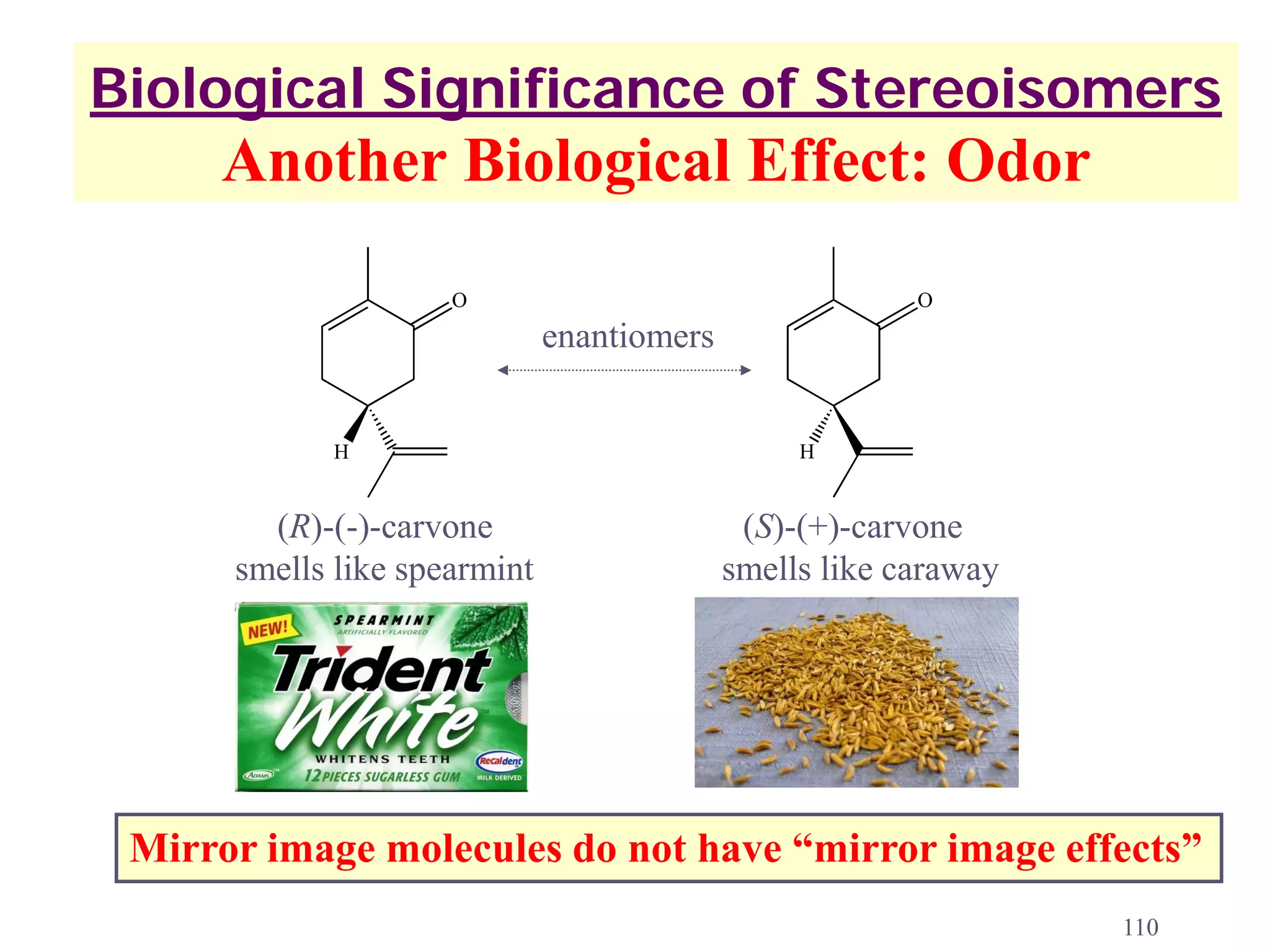
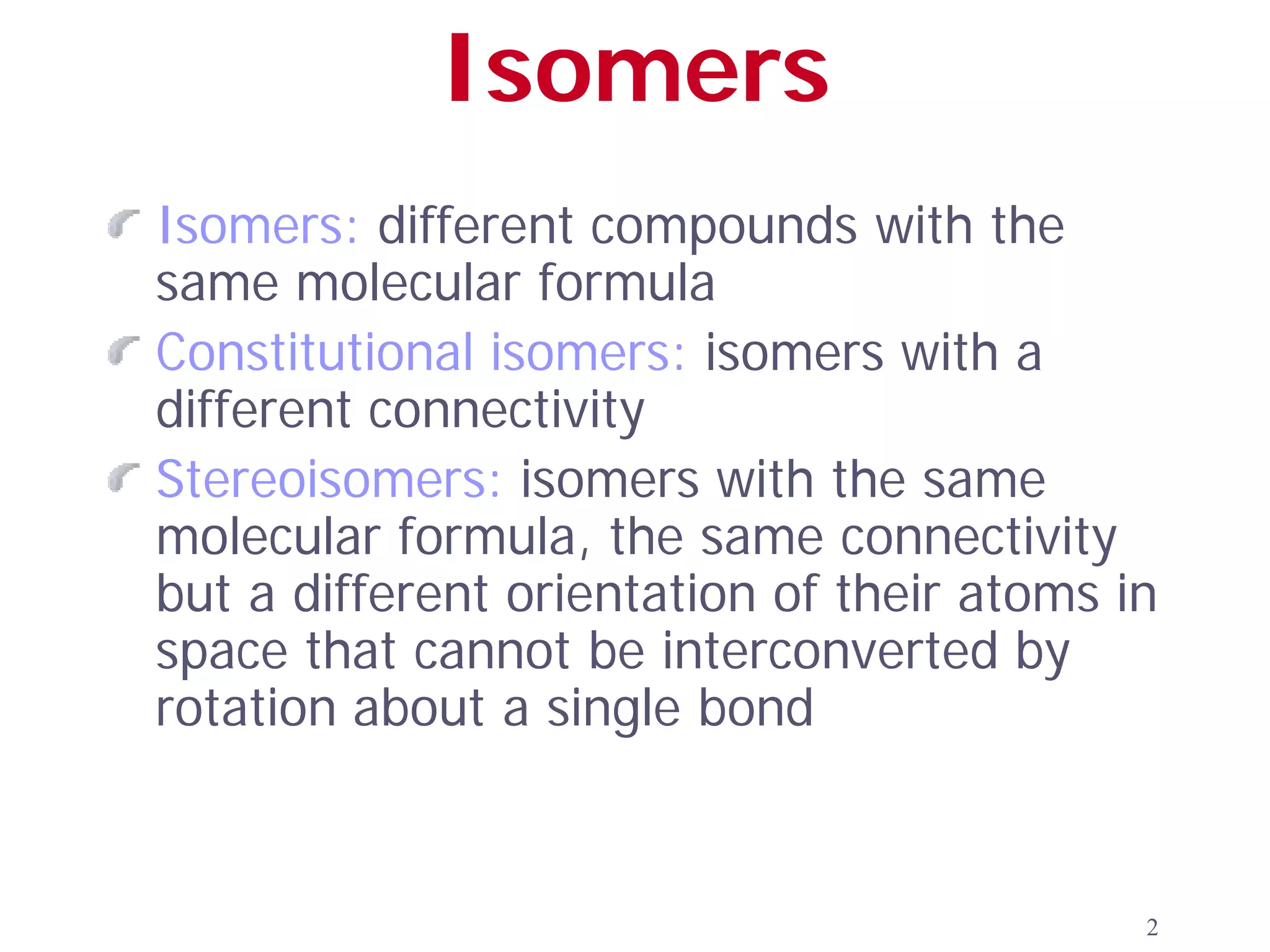
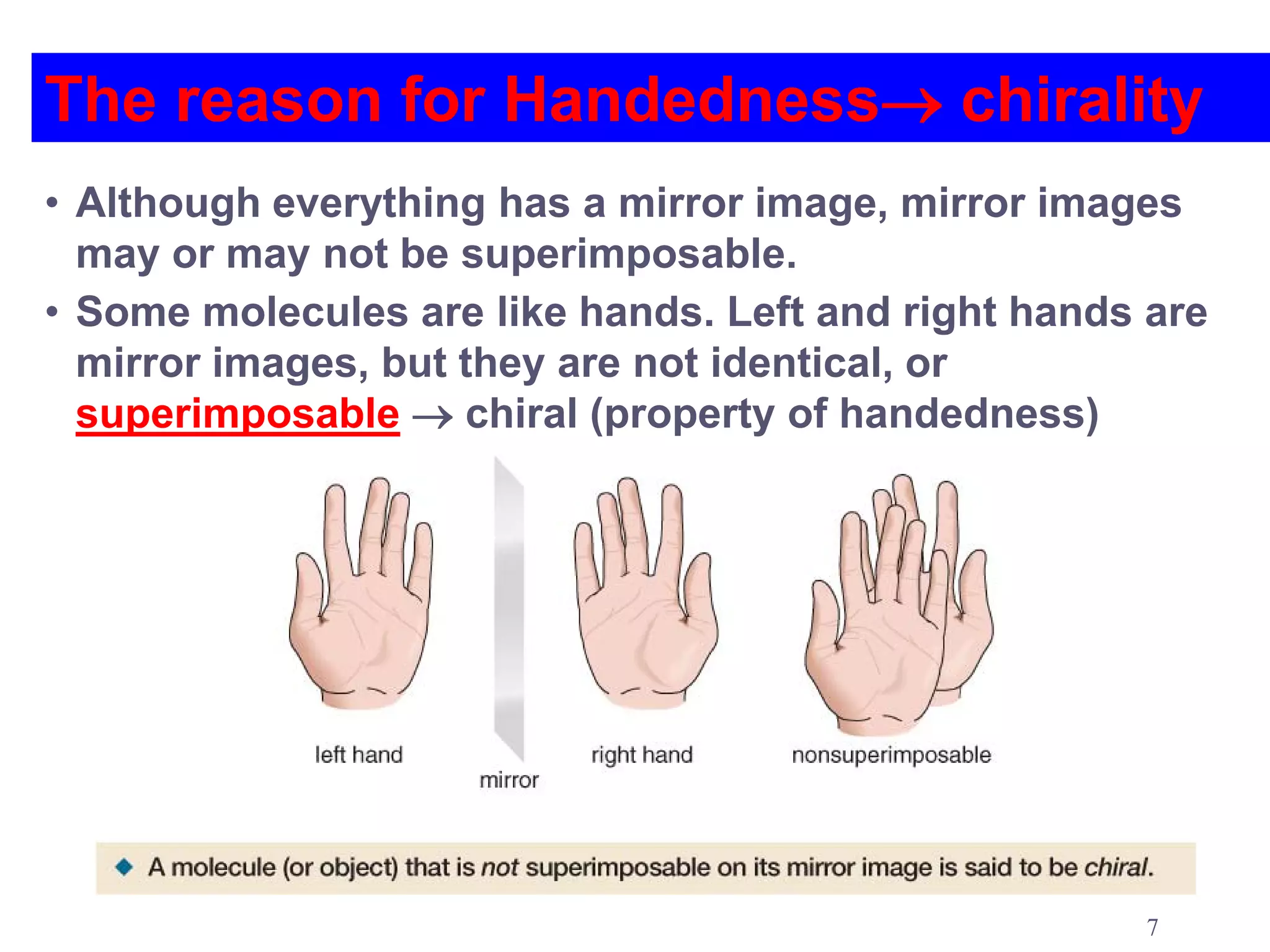
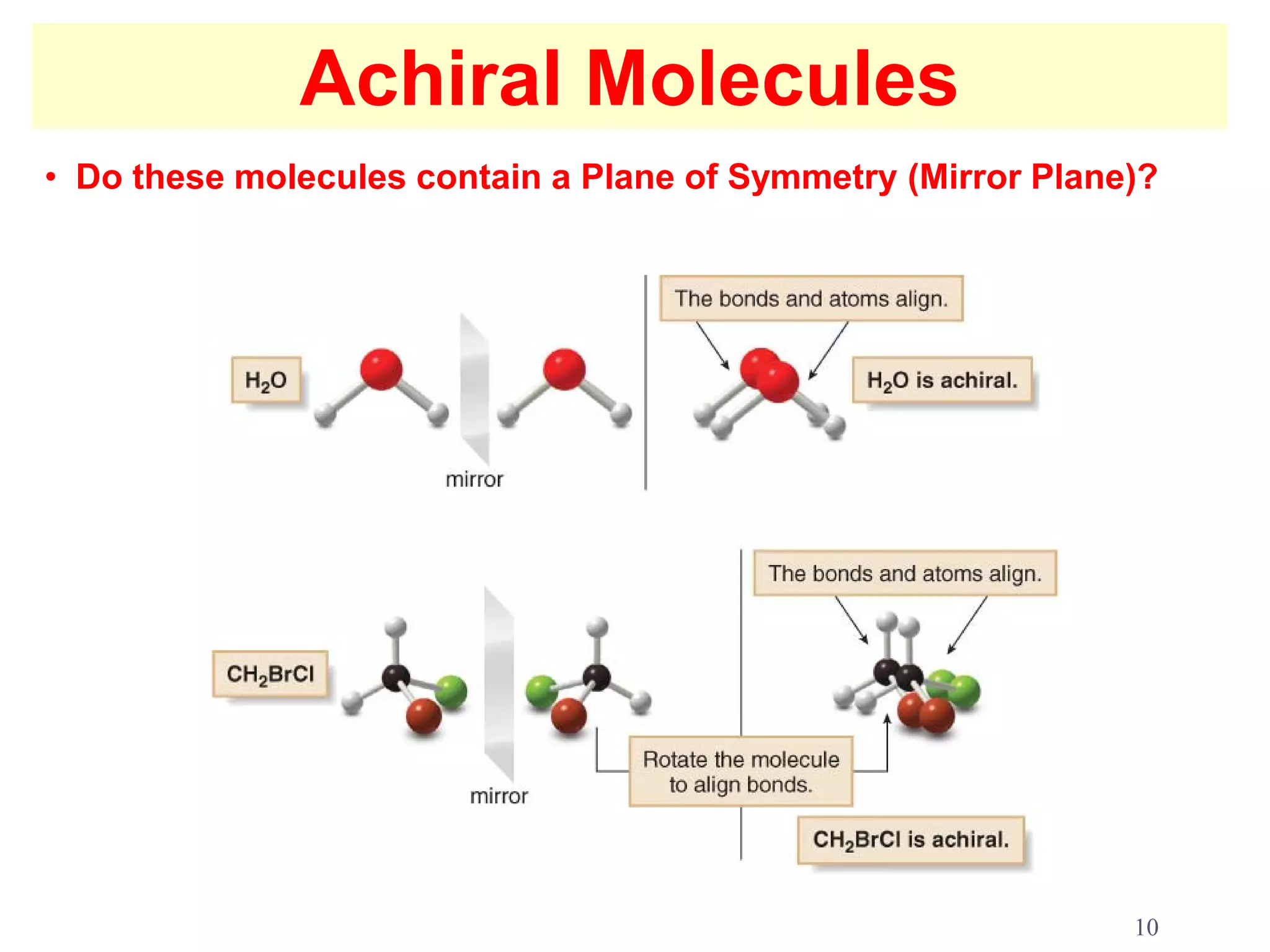
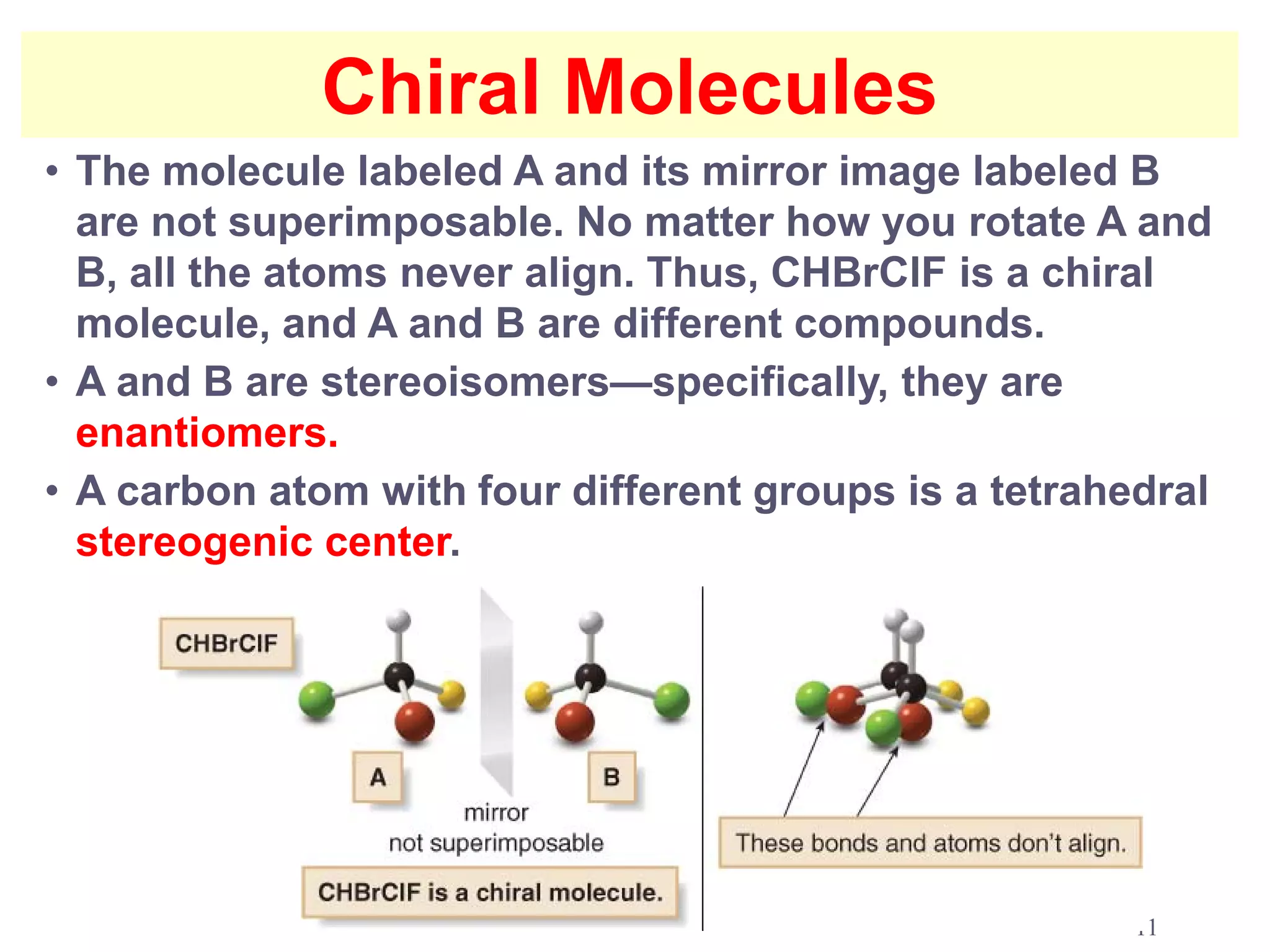
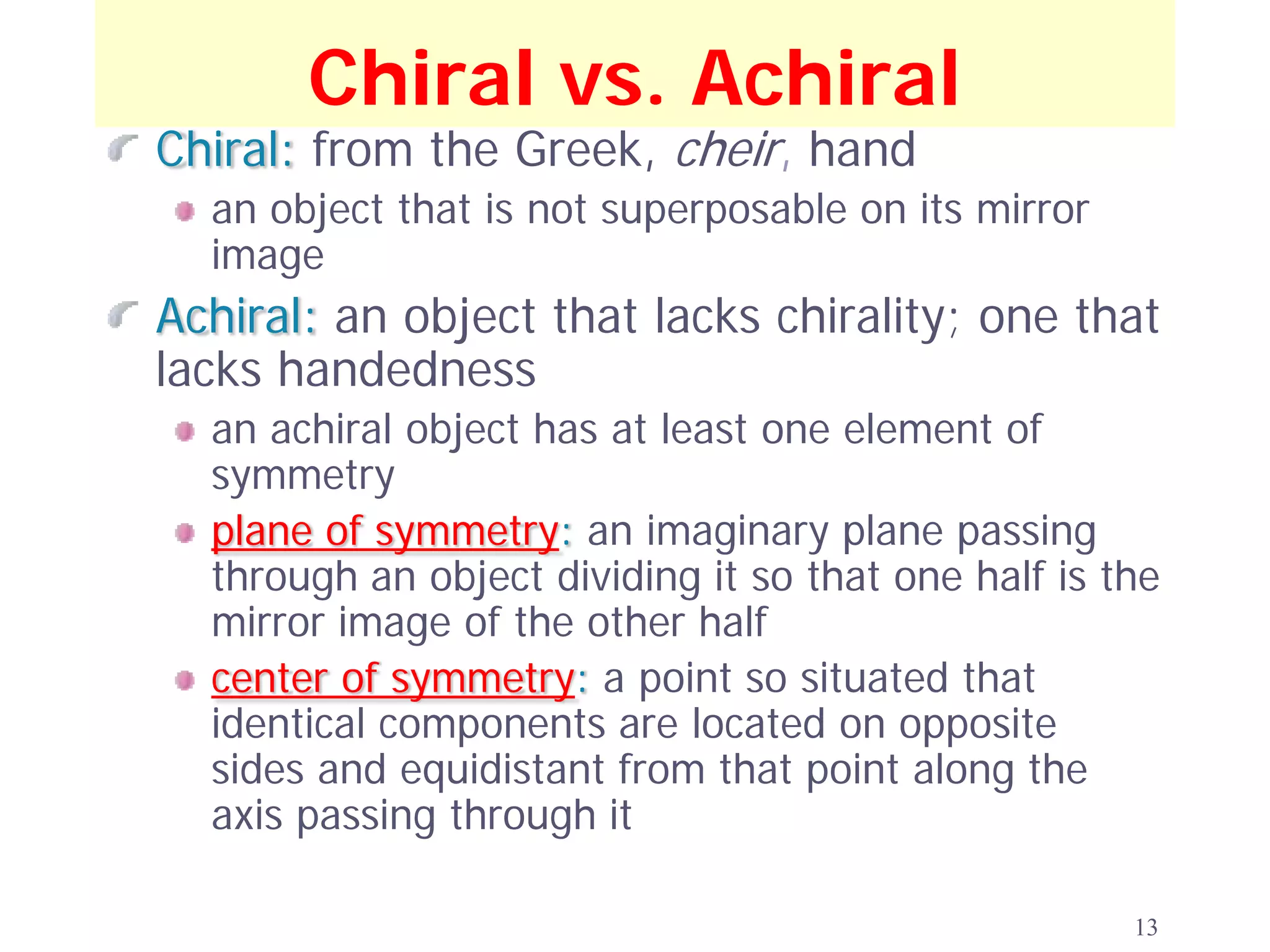
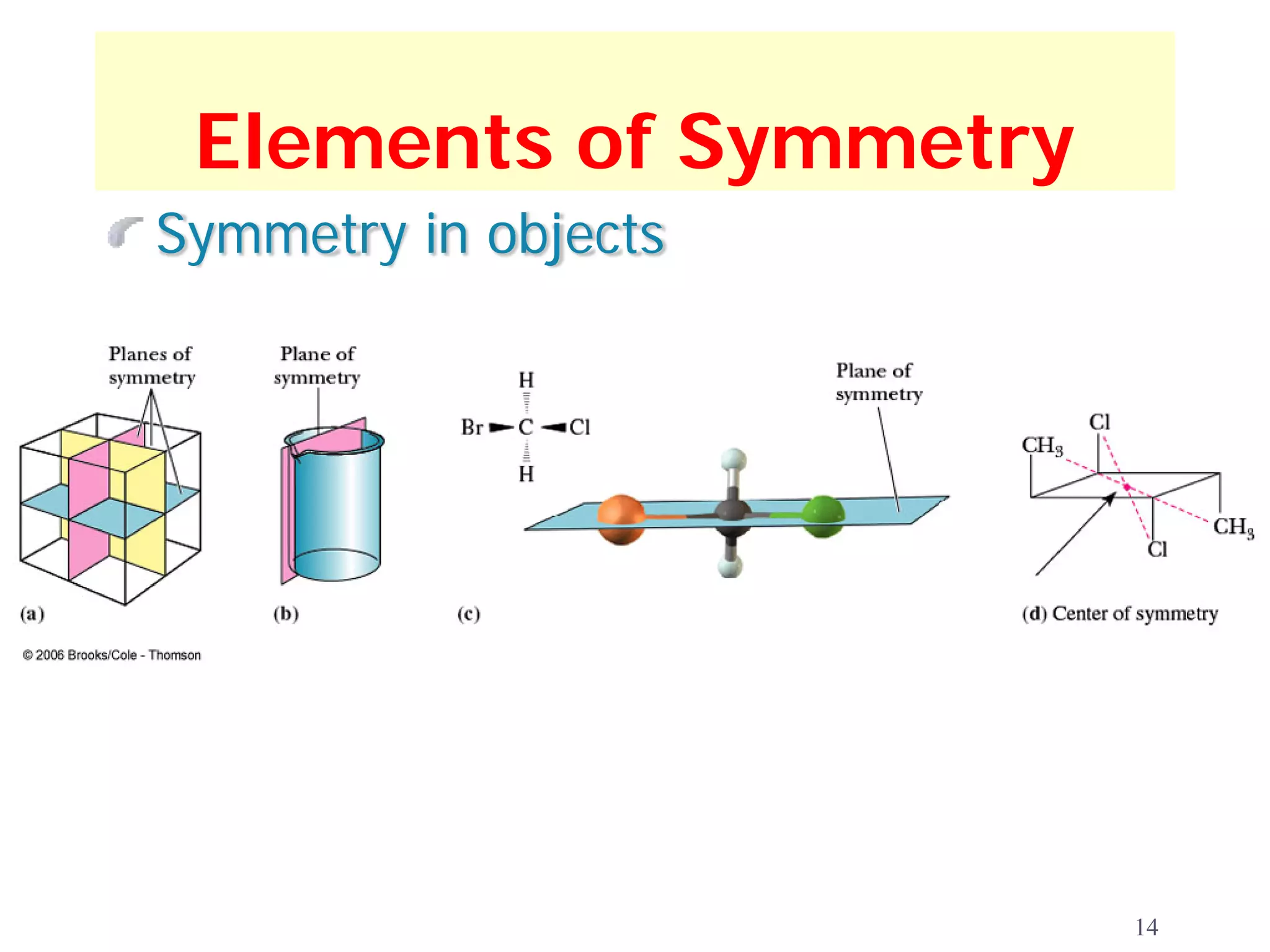
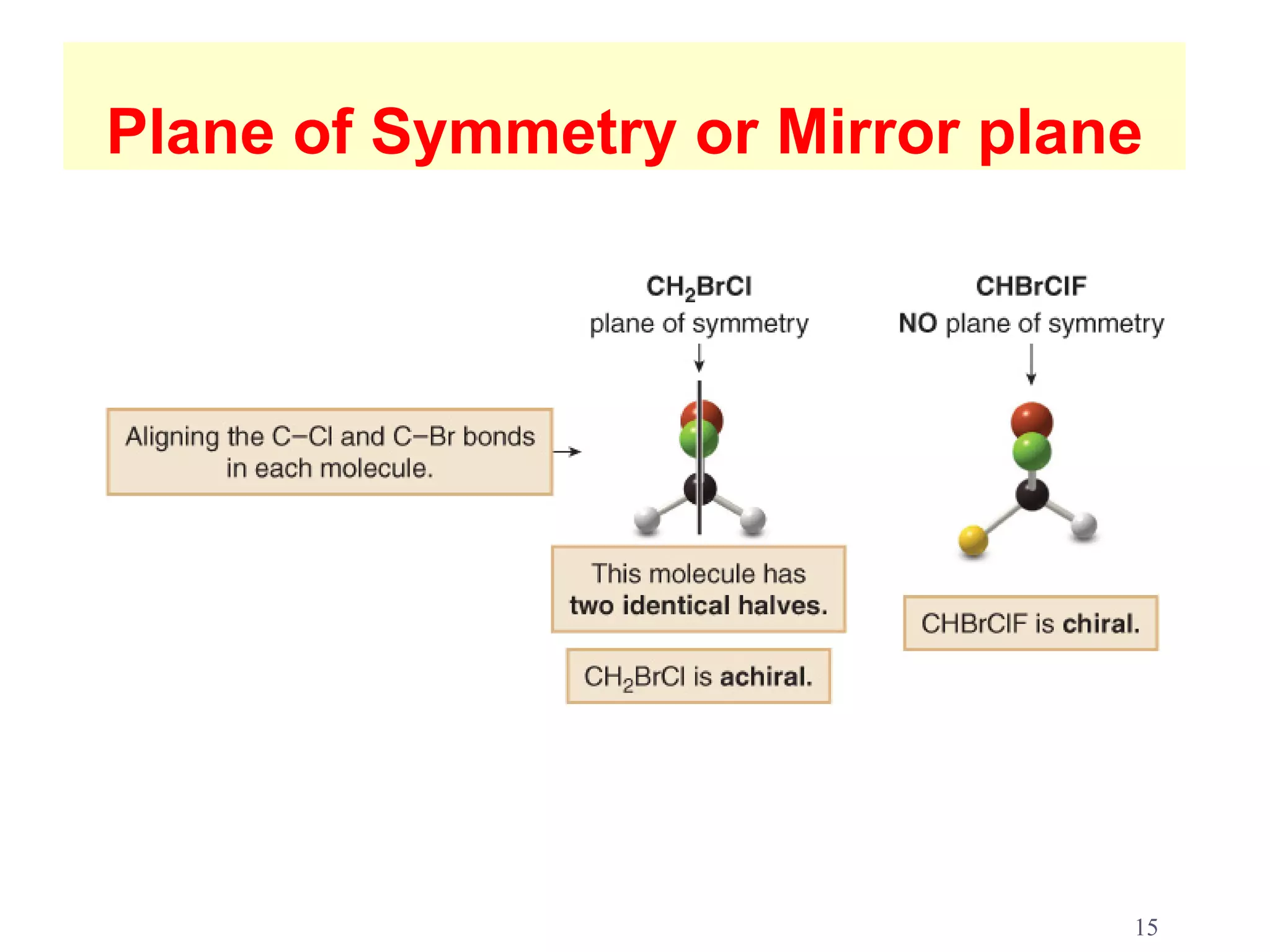
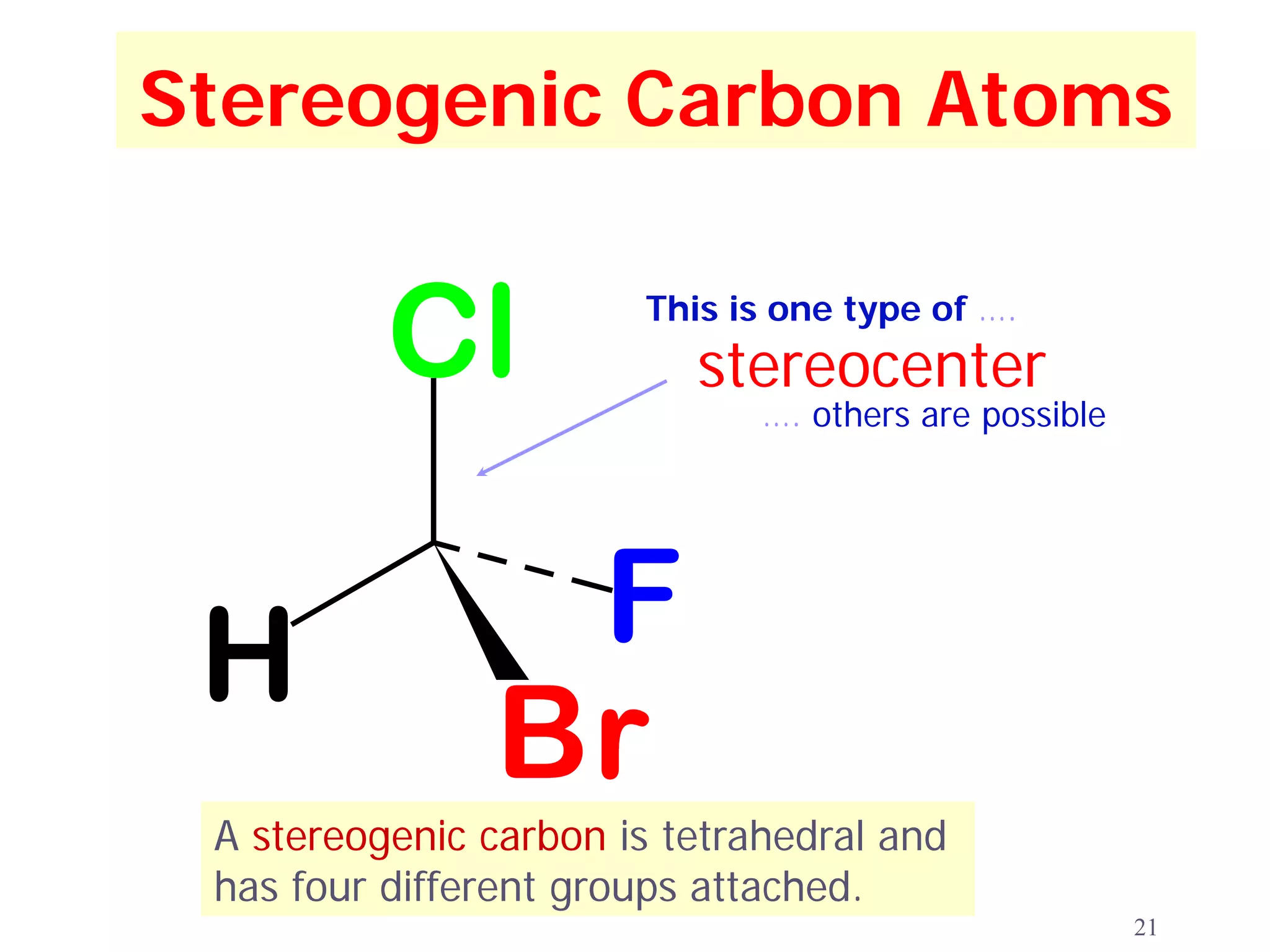
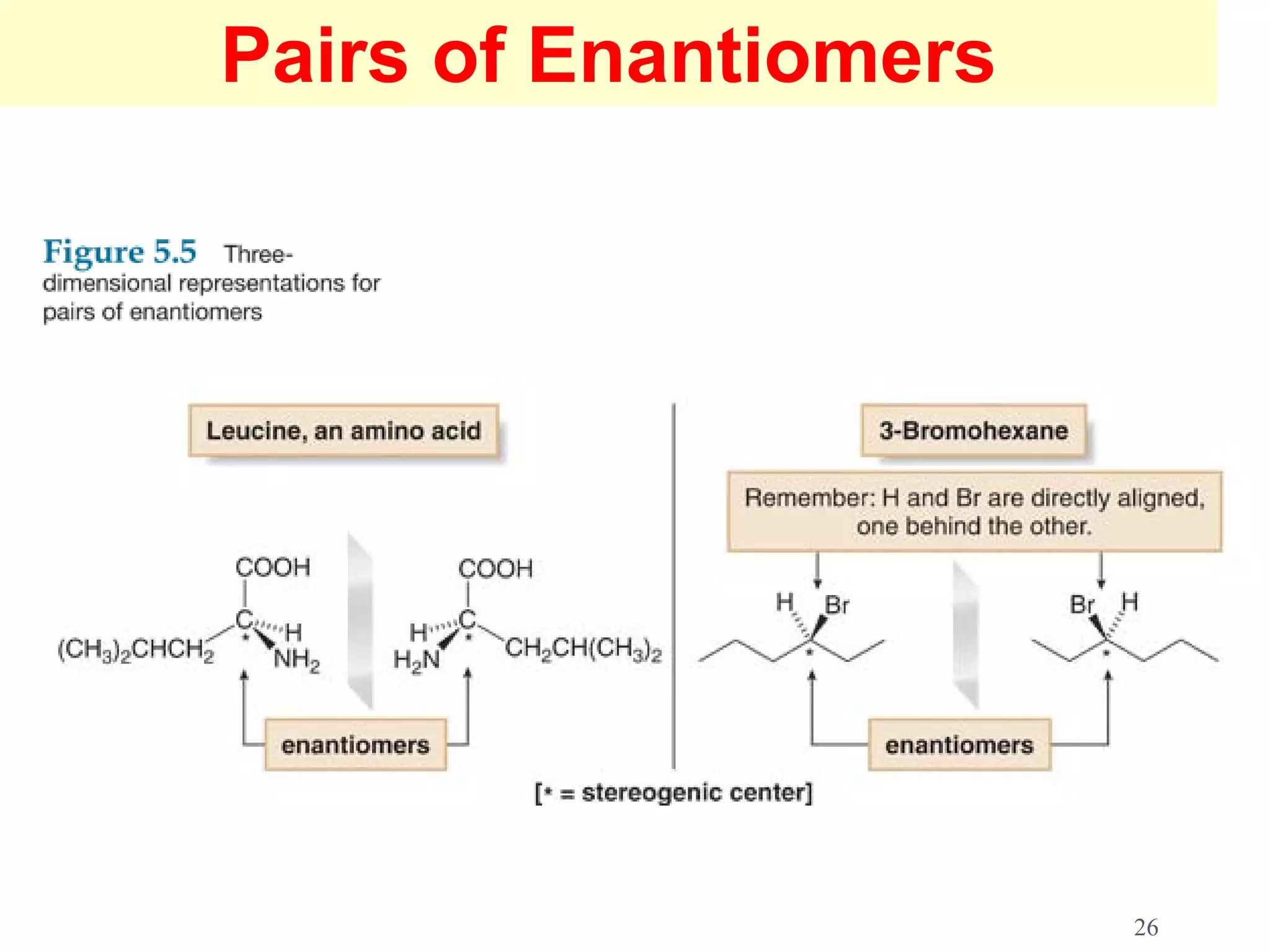
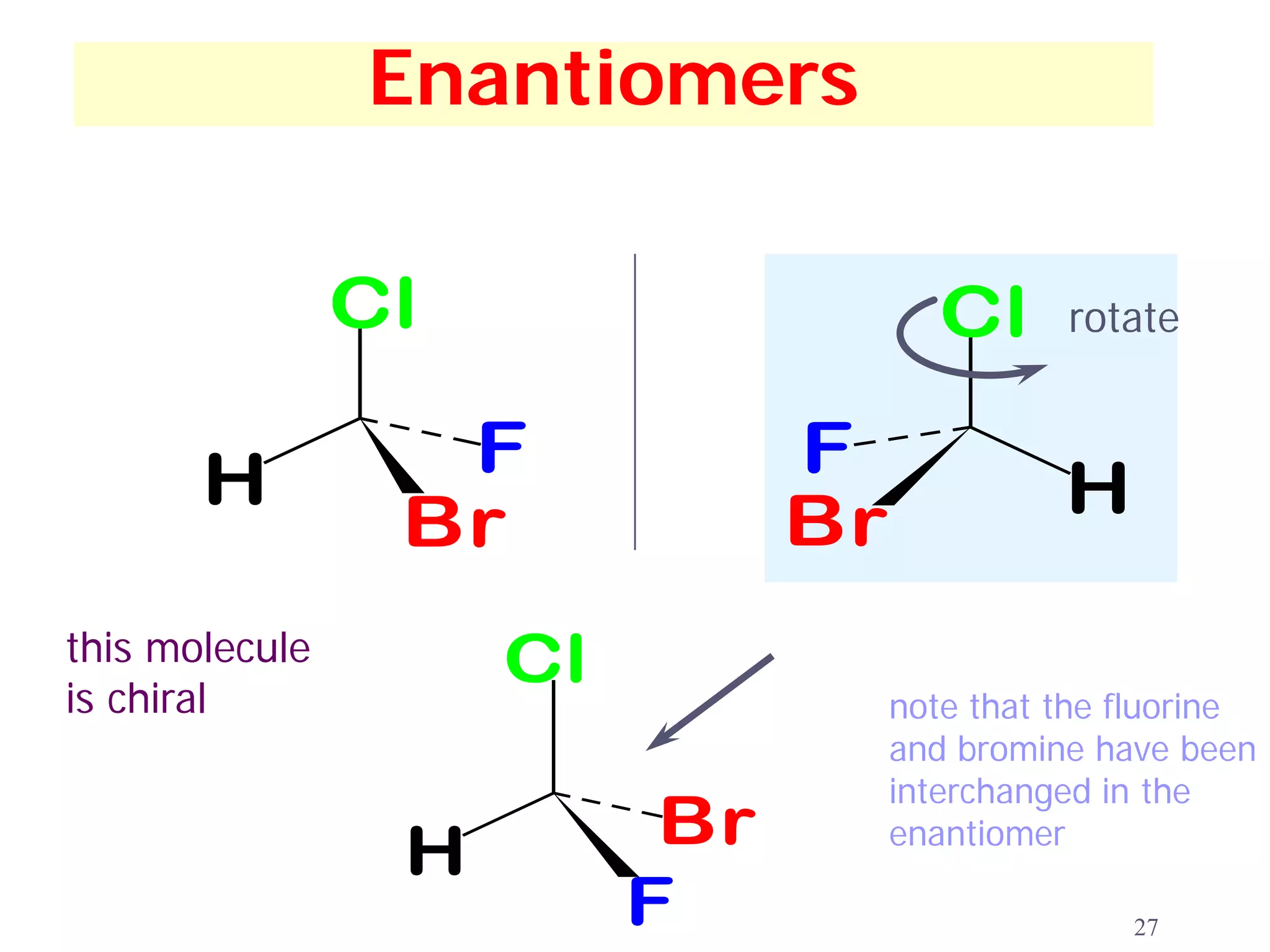
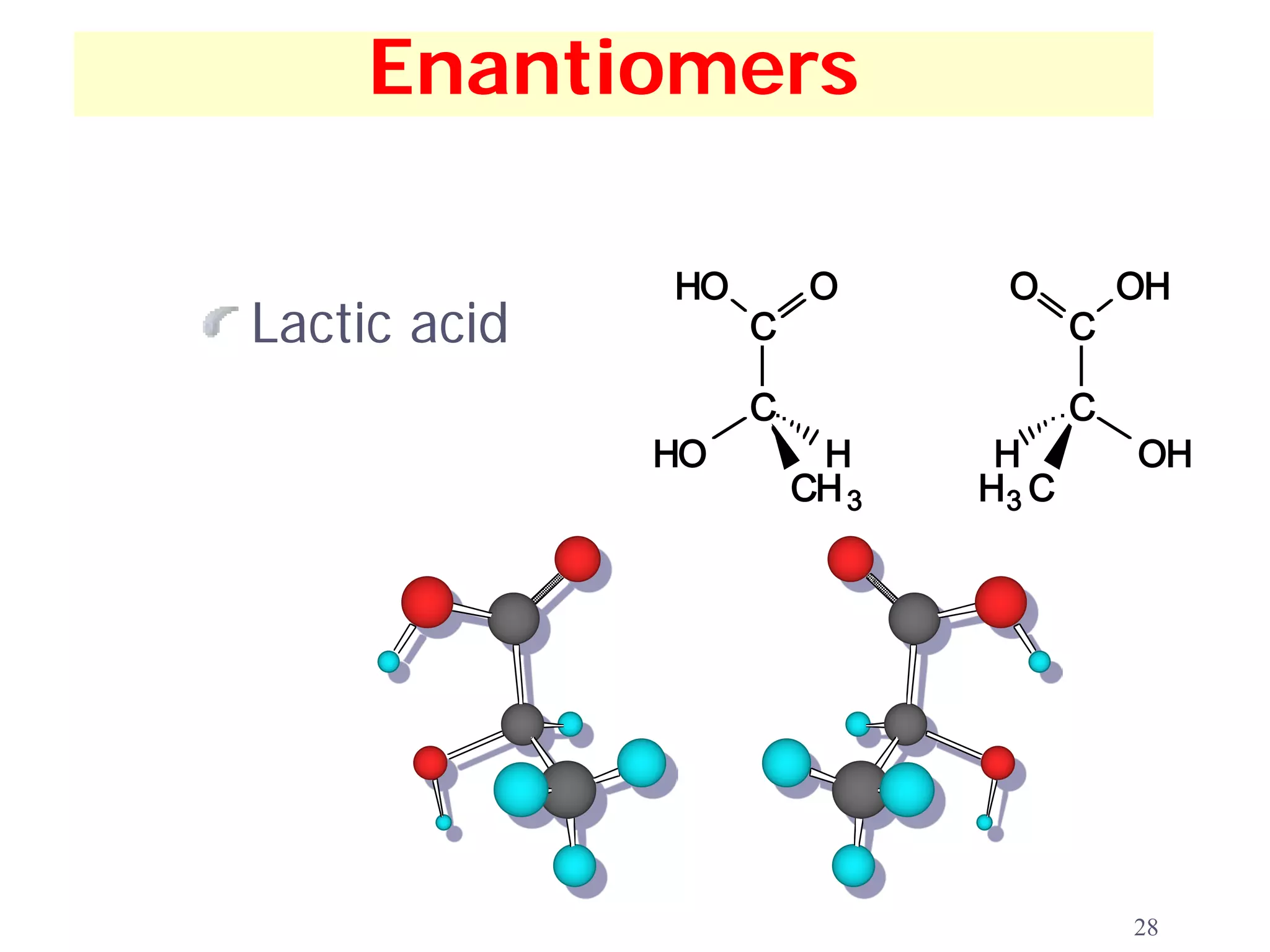
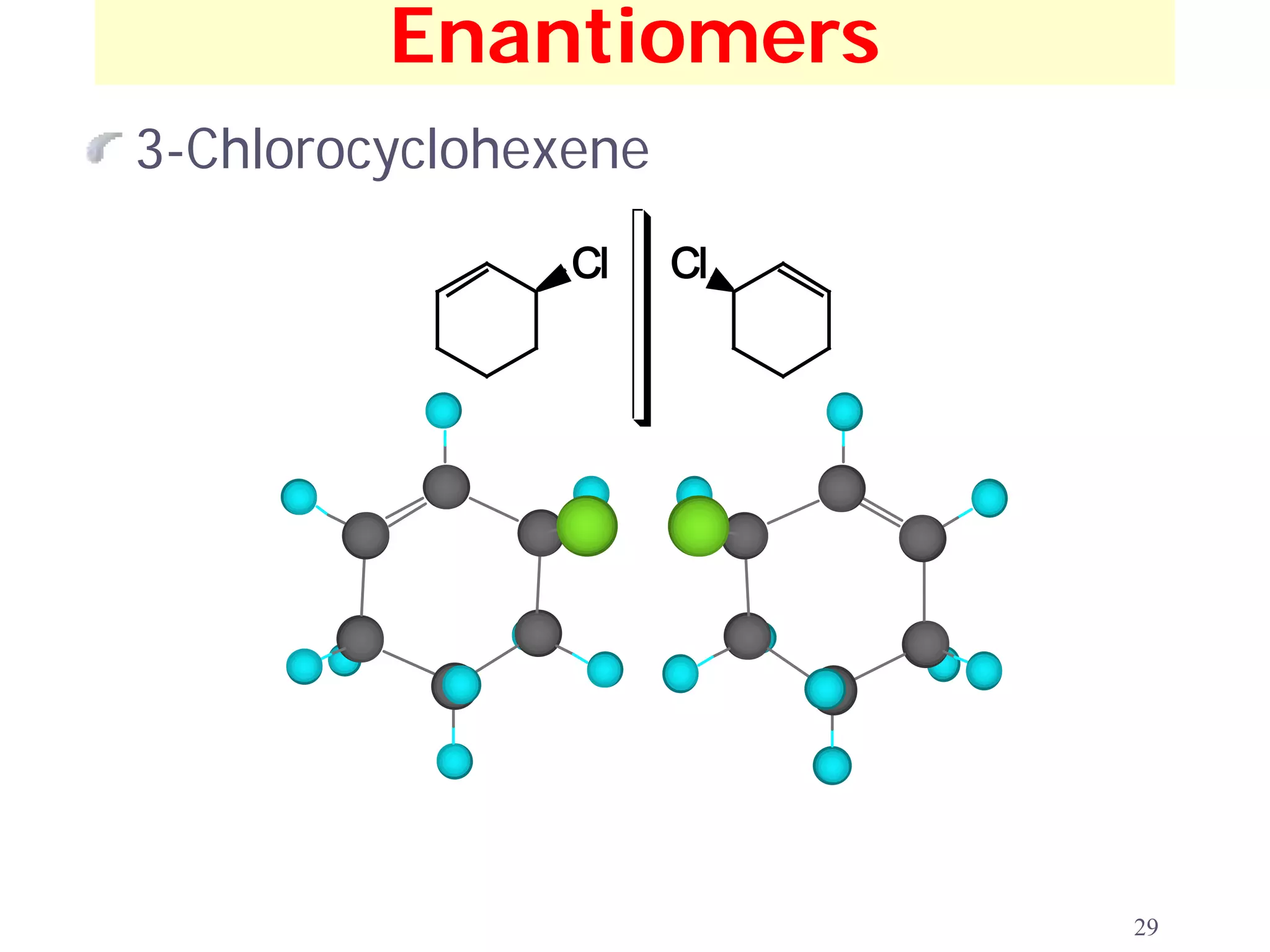
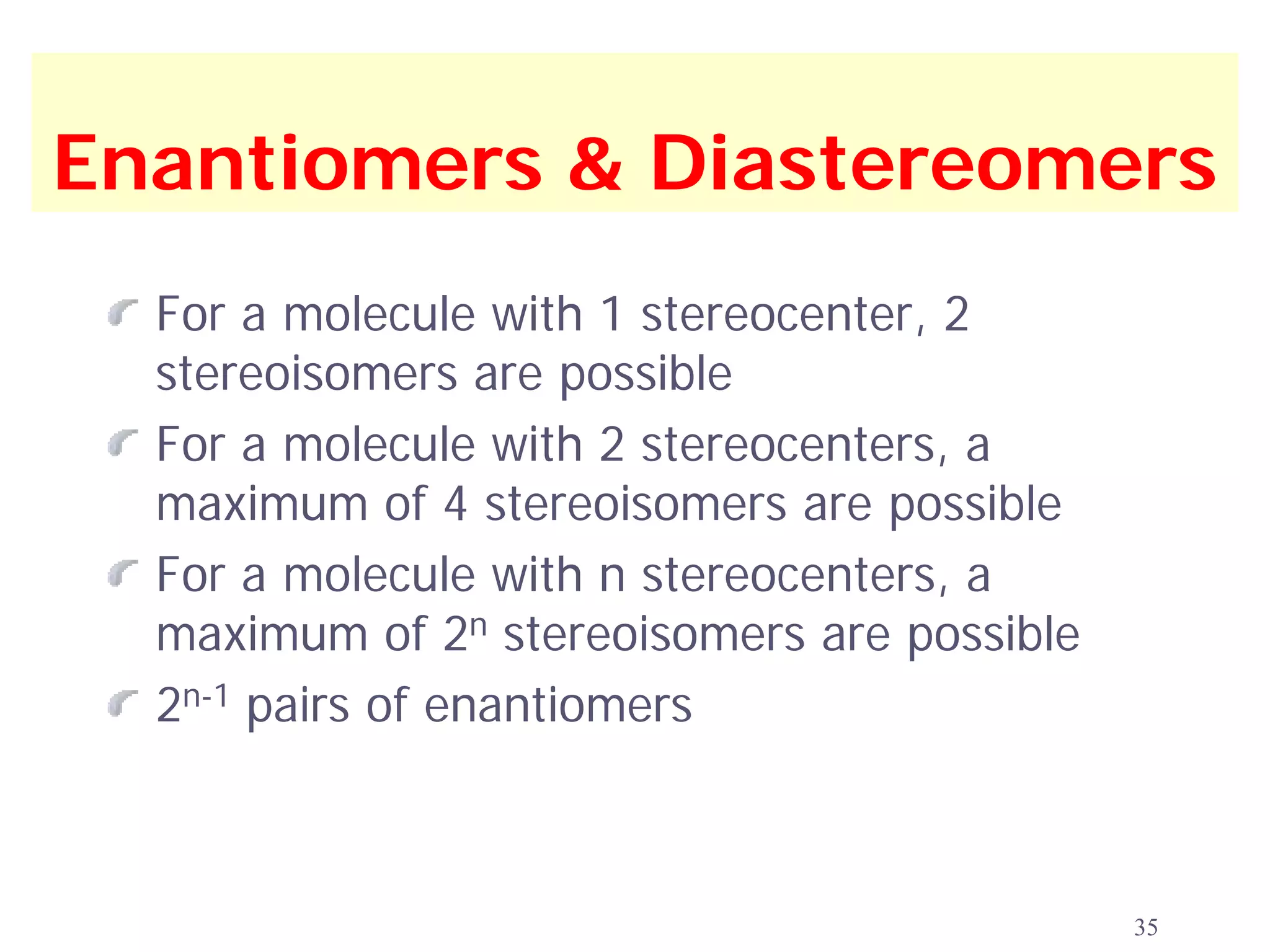
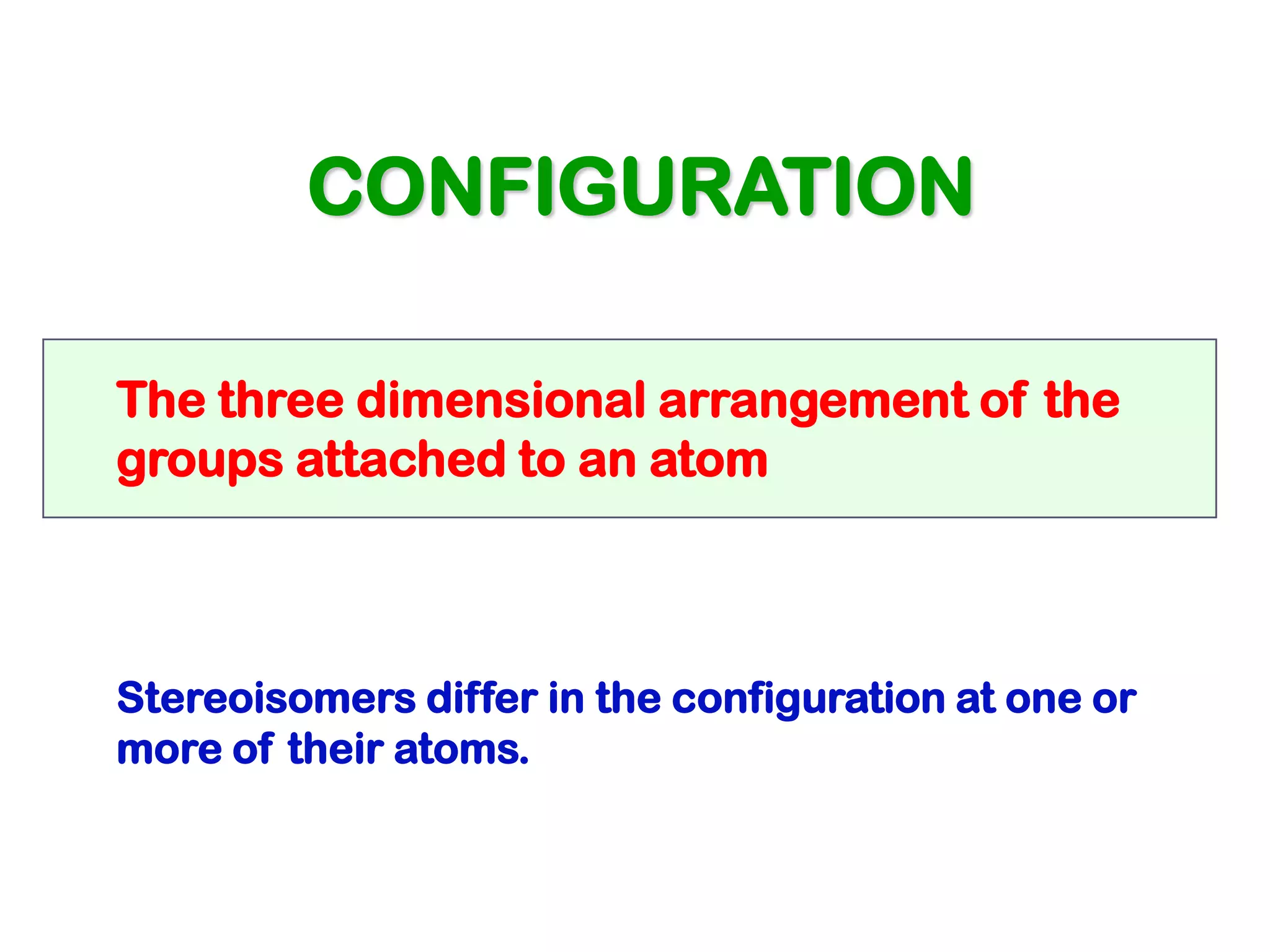
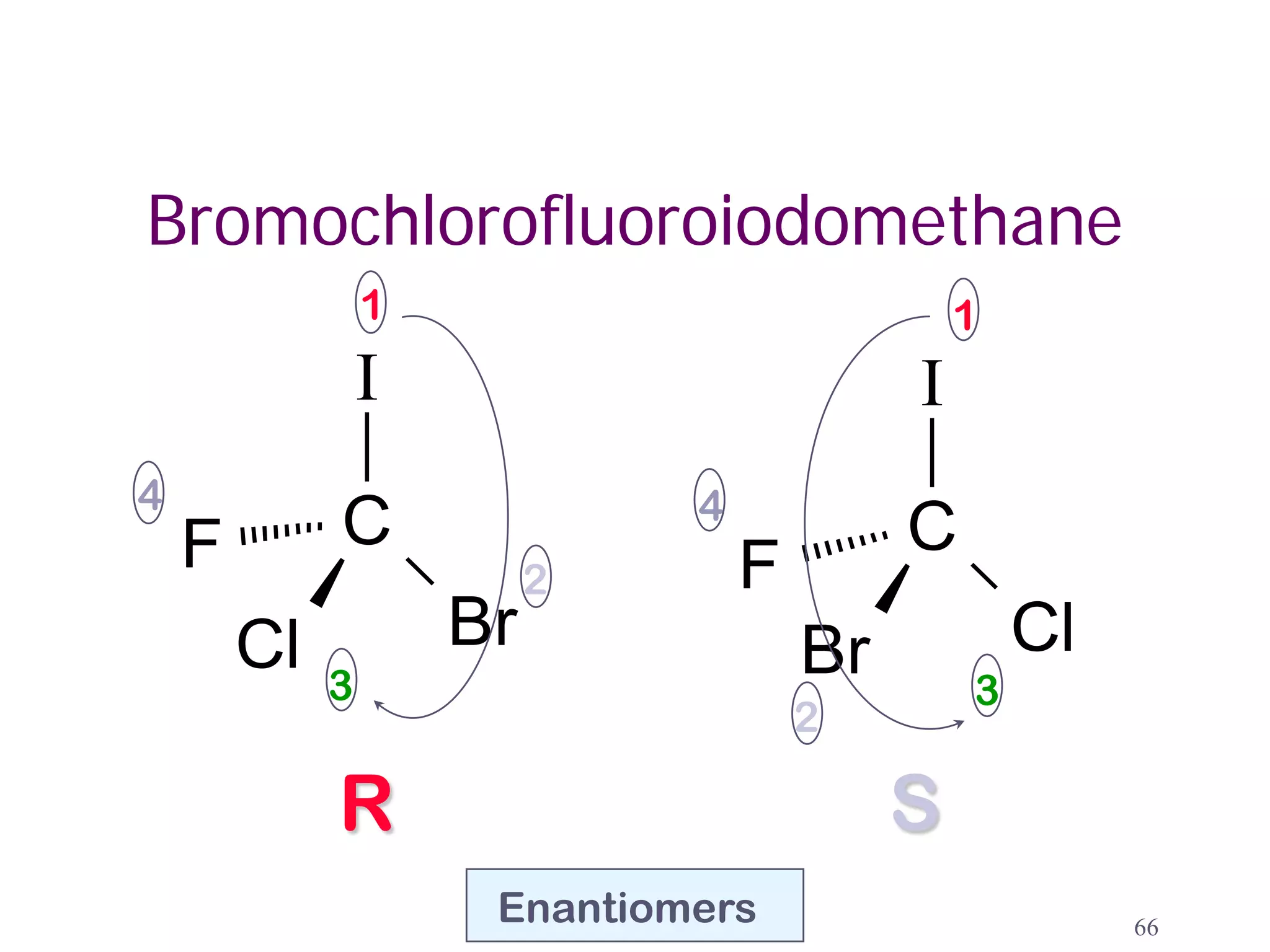
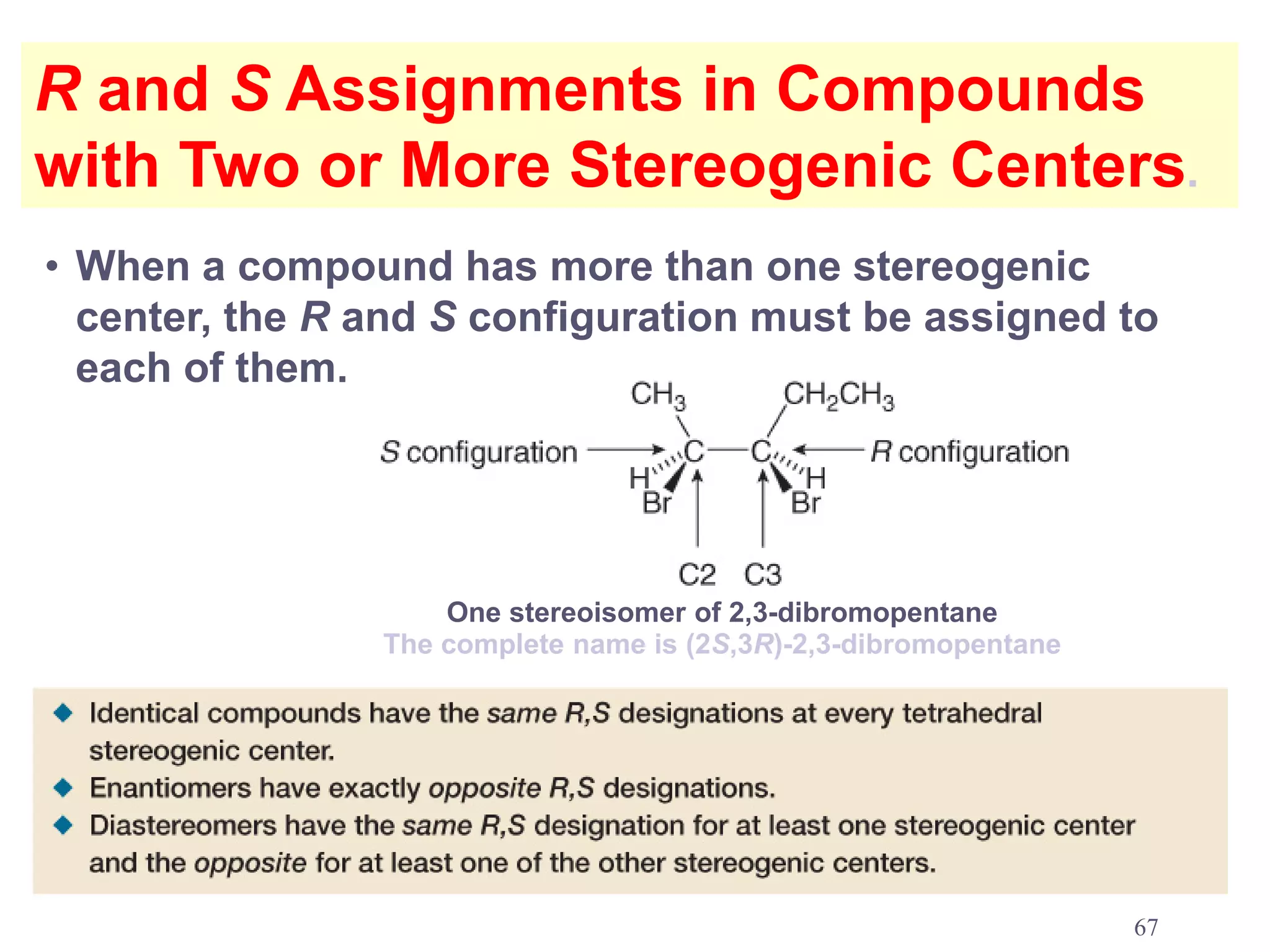
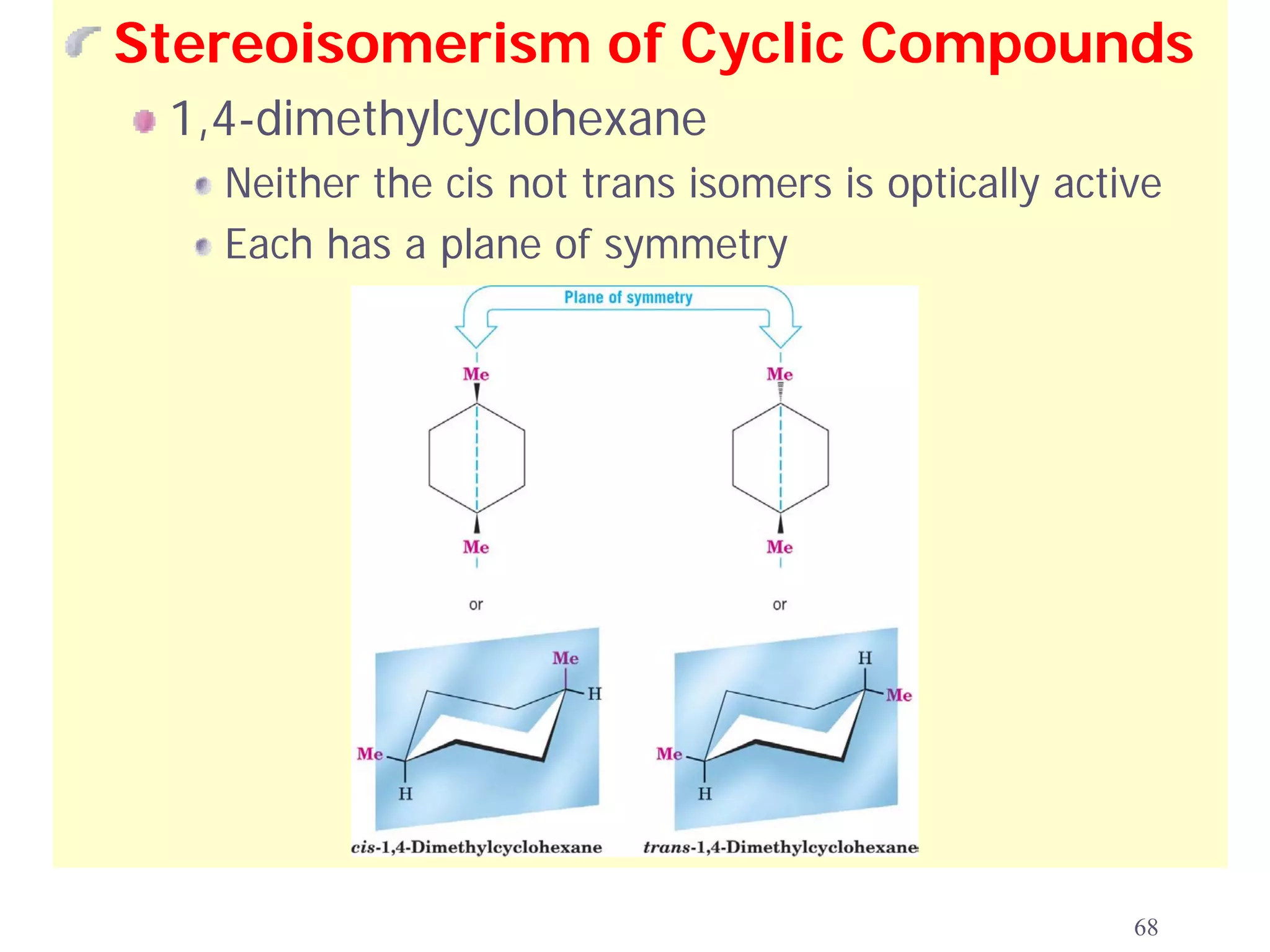
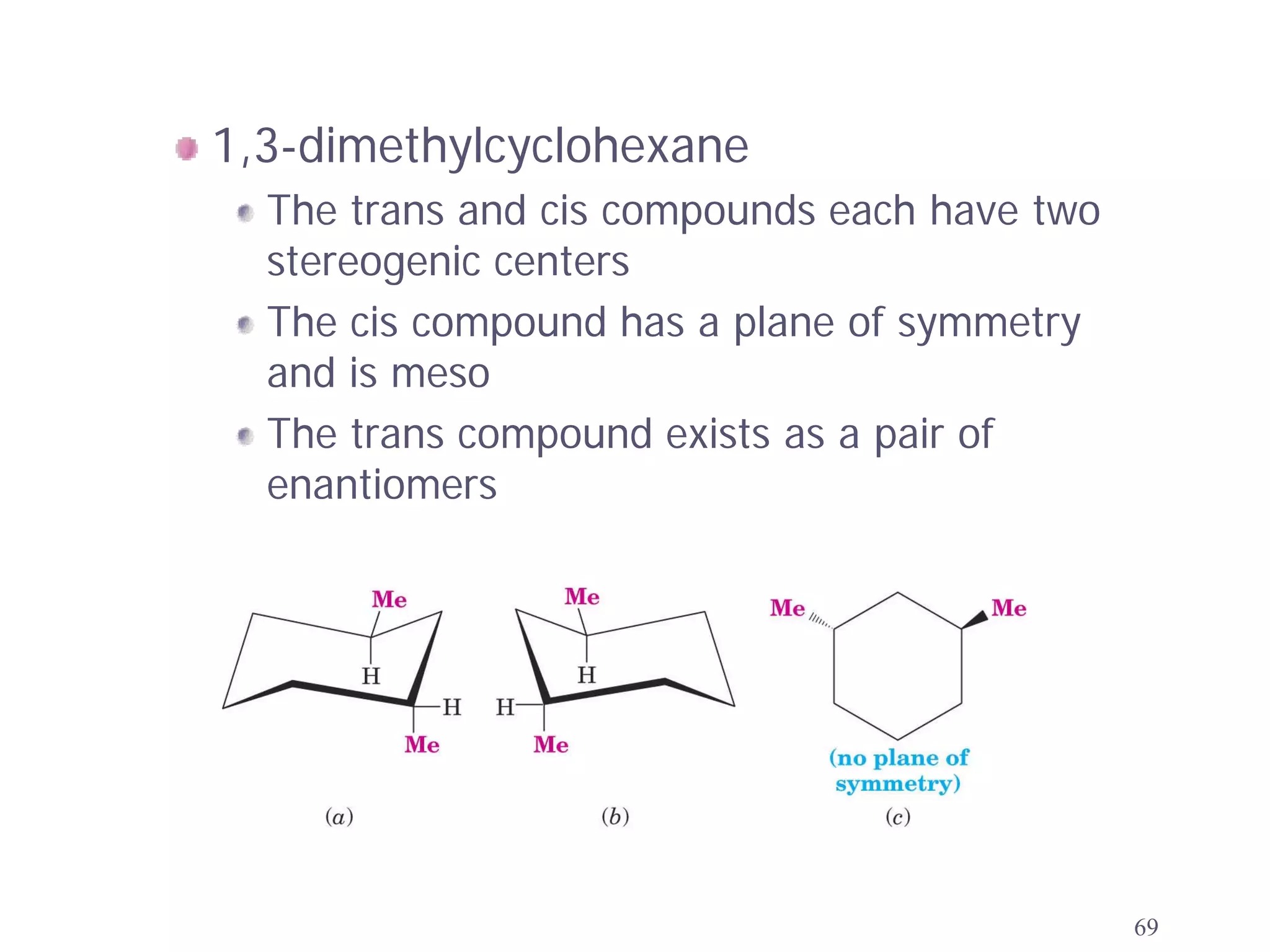
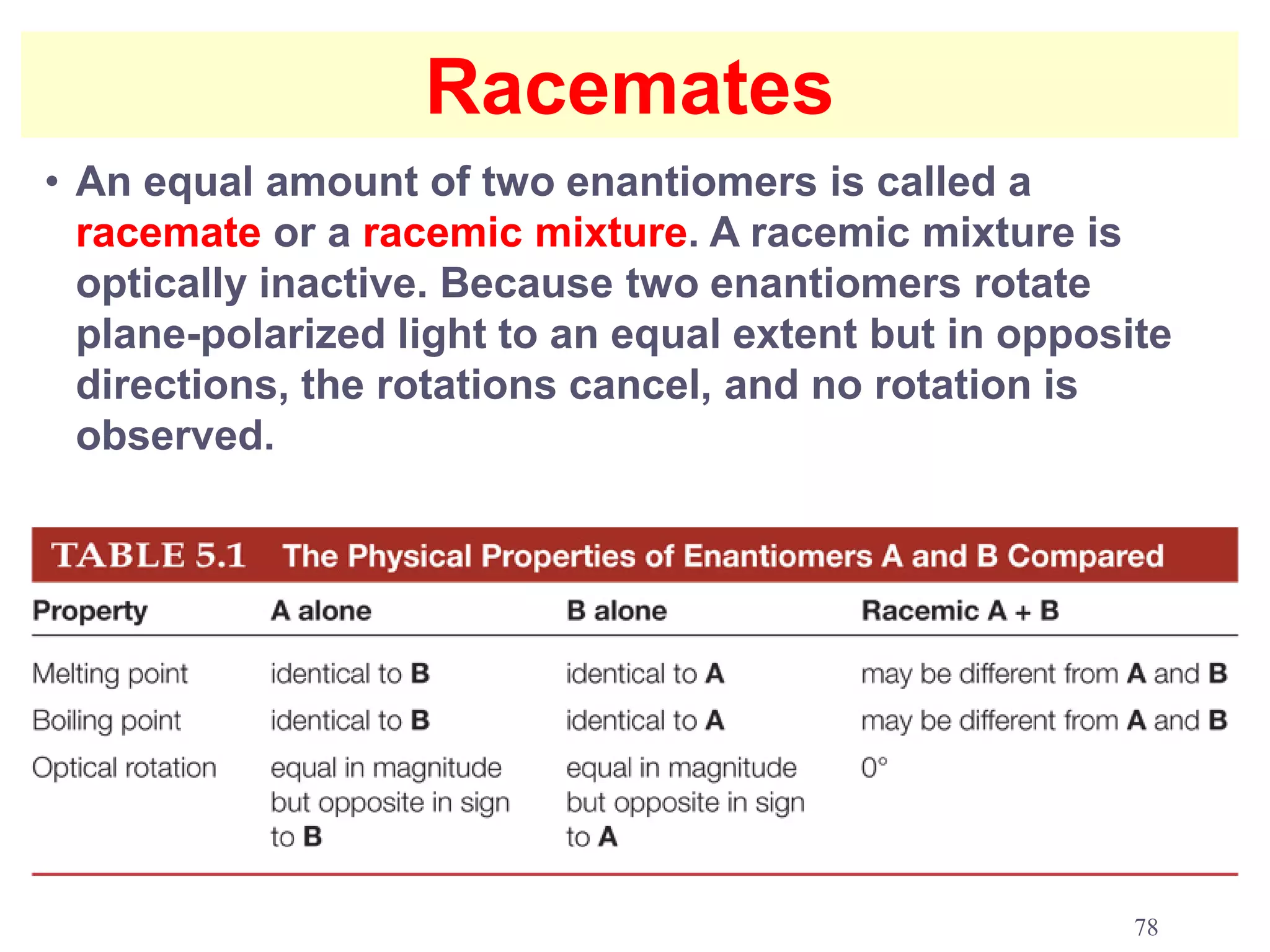
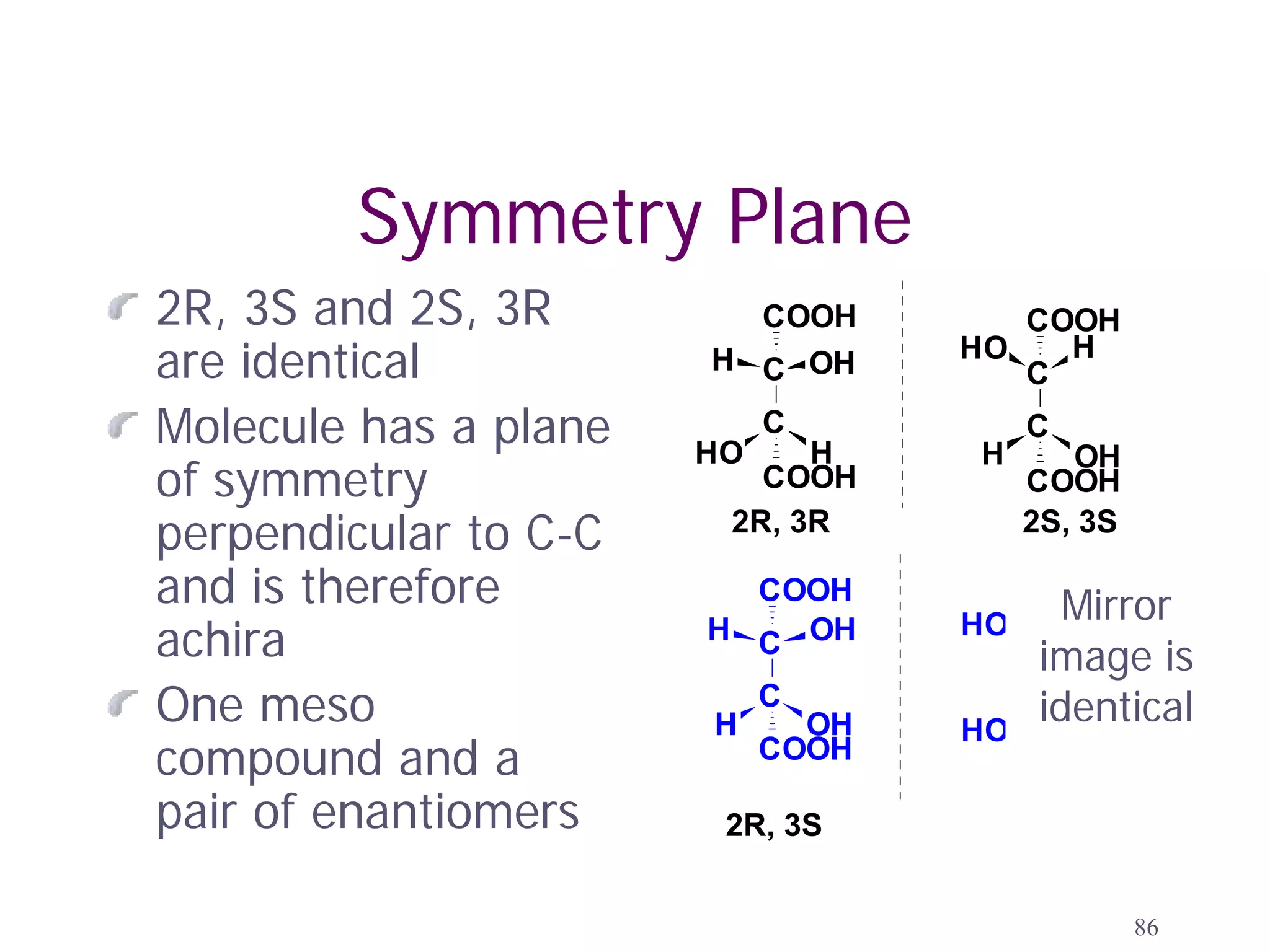
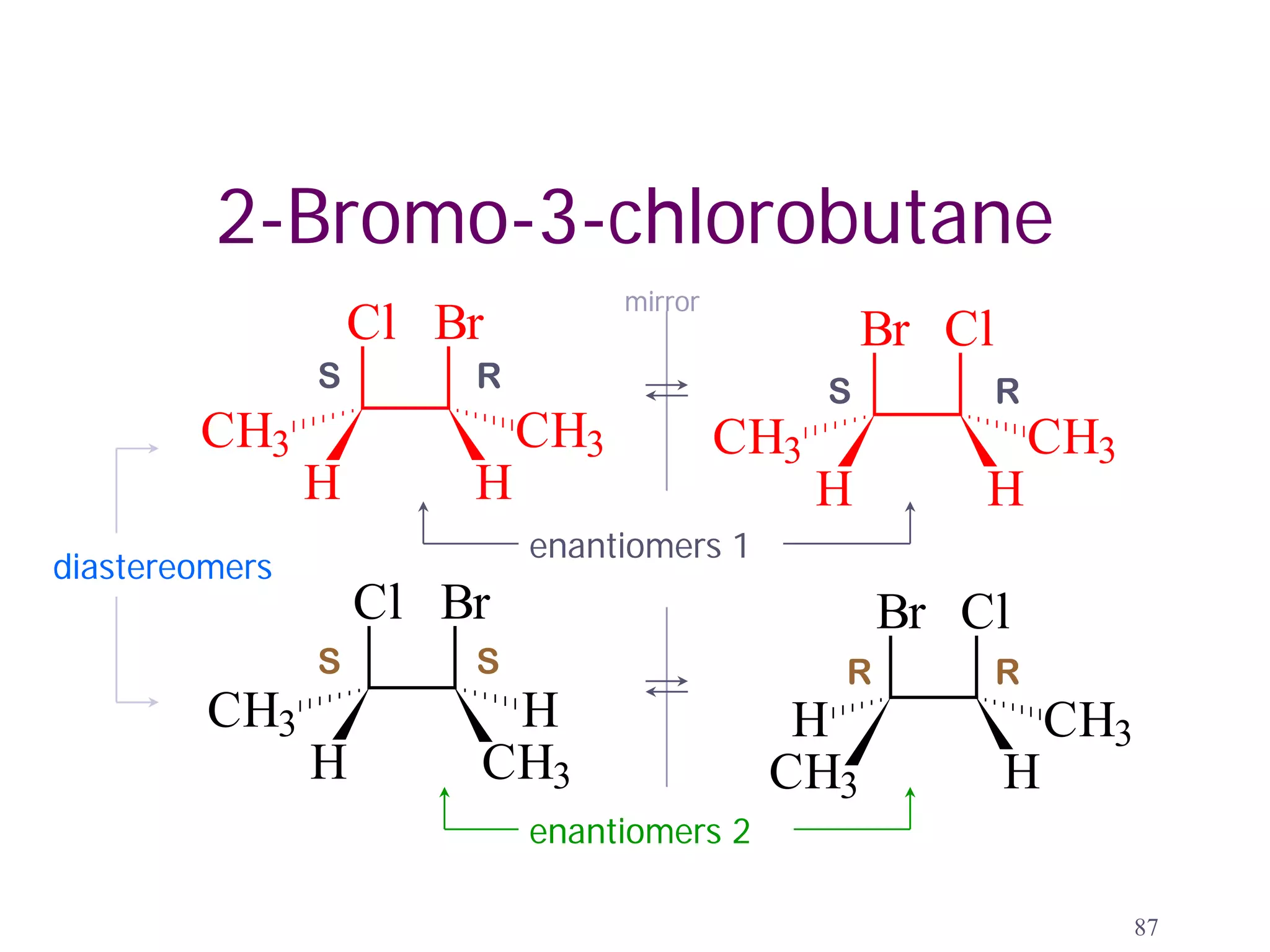
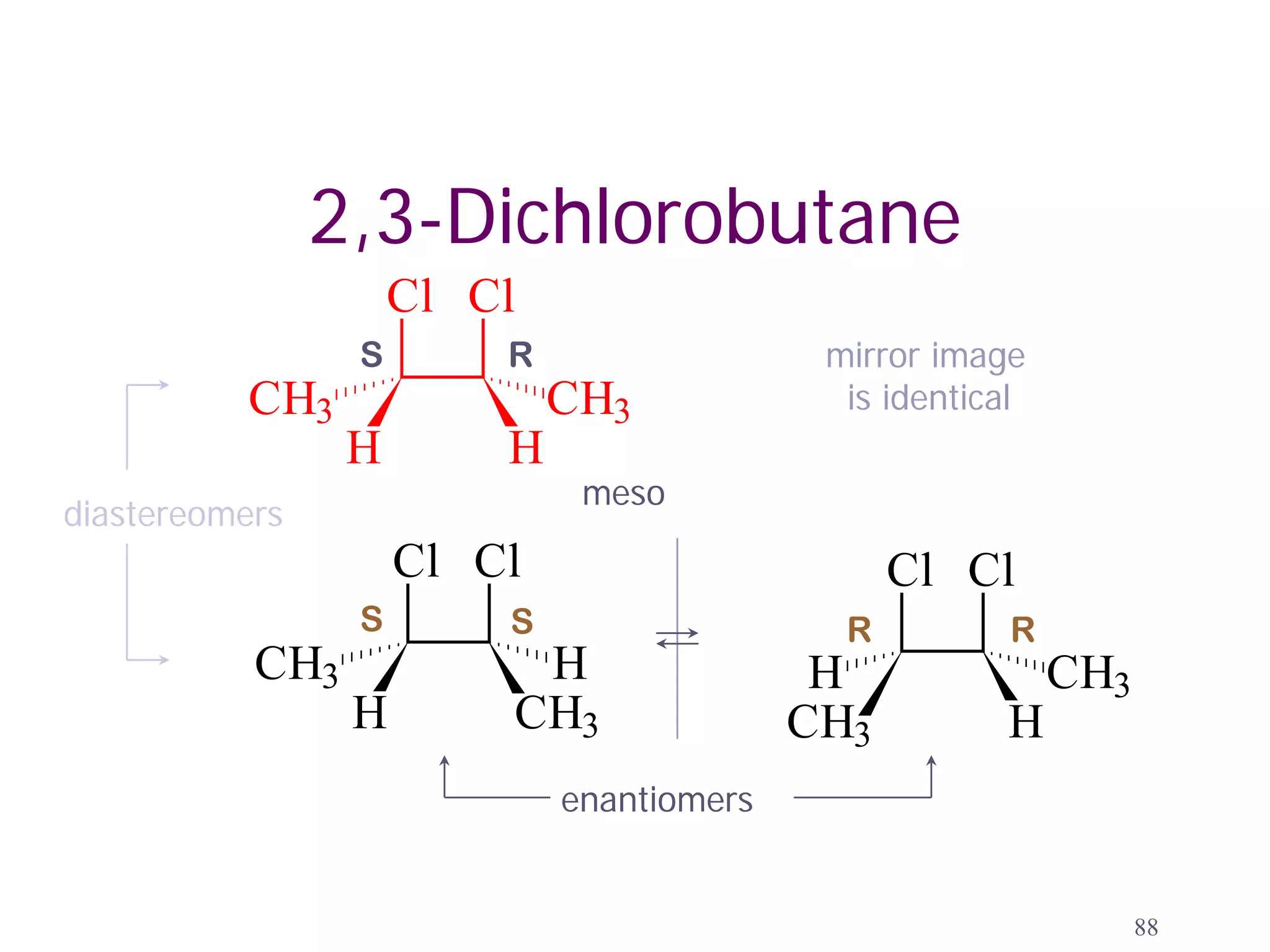
1. The document discusses isomers, stereochemistry, chirality, handedness, and the Cahn-Ingold-Prelog system for assigning R and S configurations to stereogenic centers.
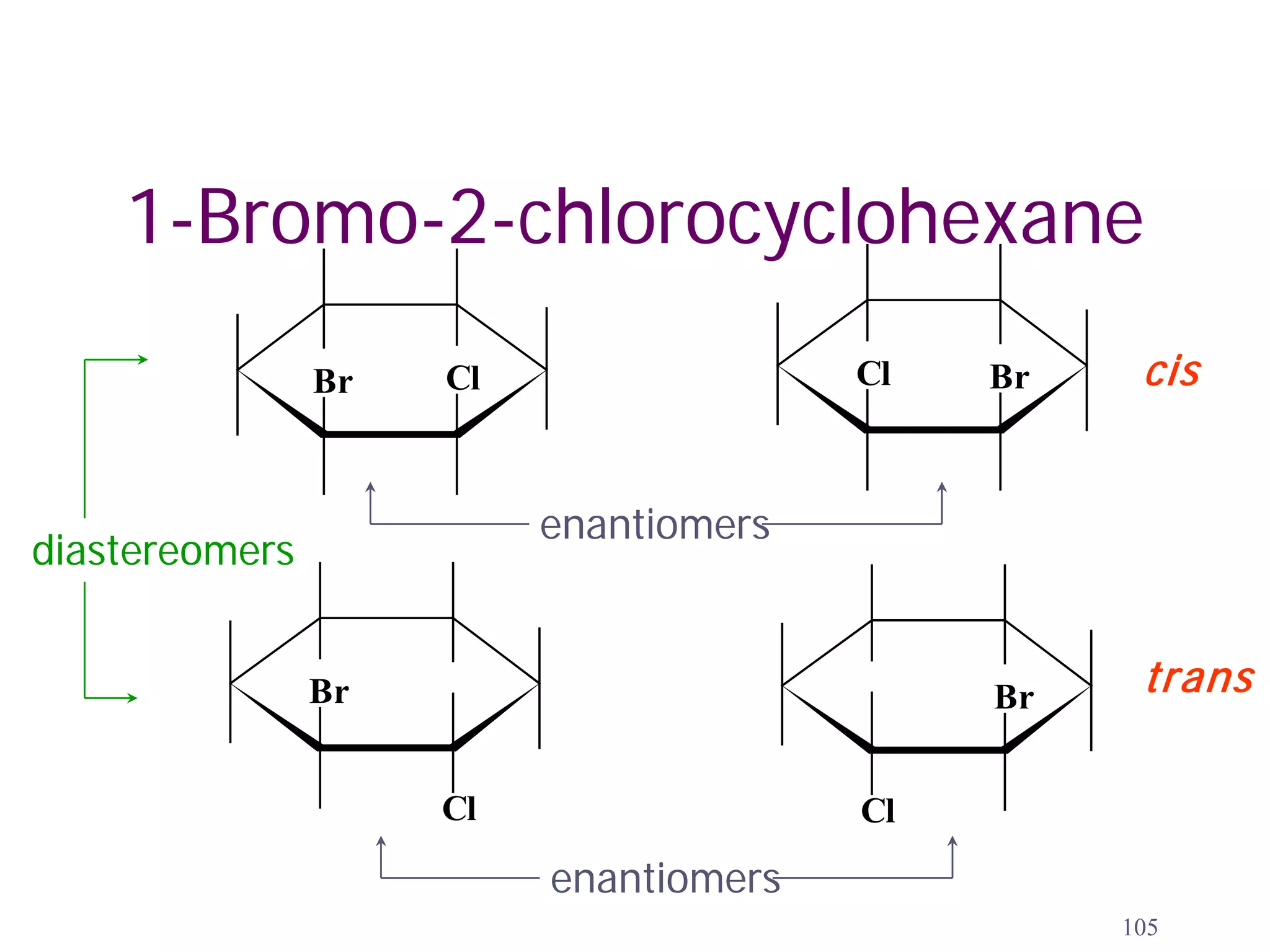
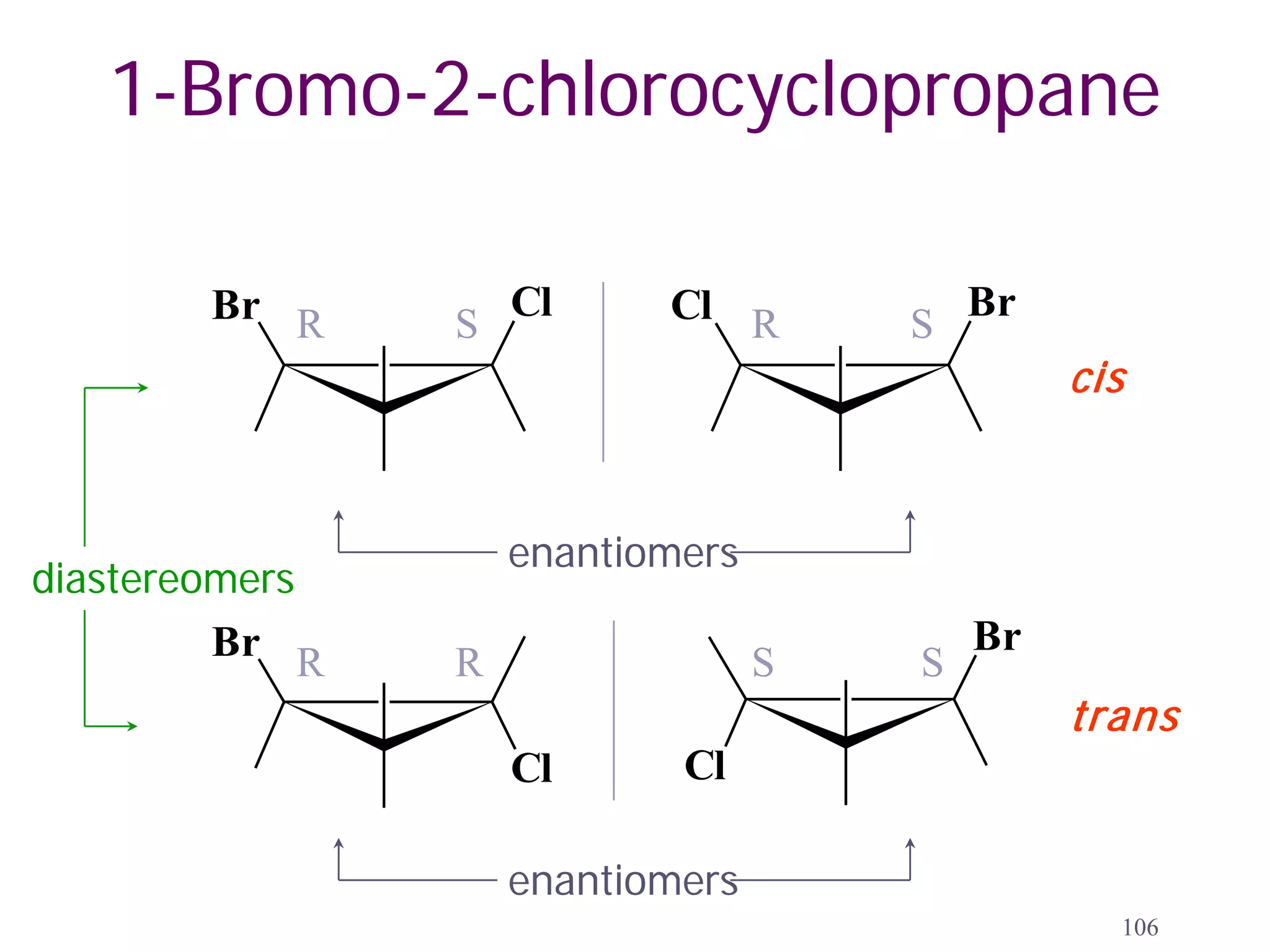
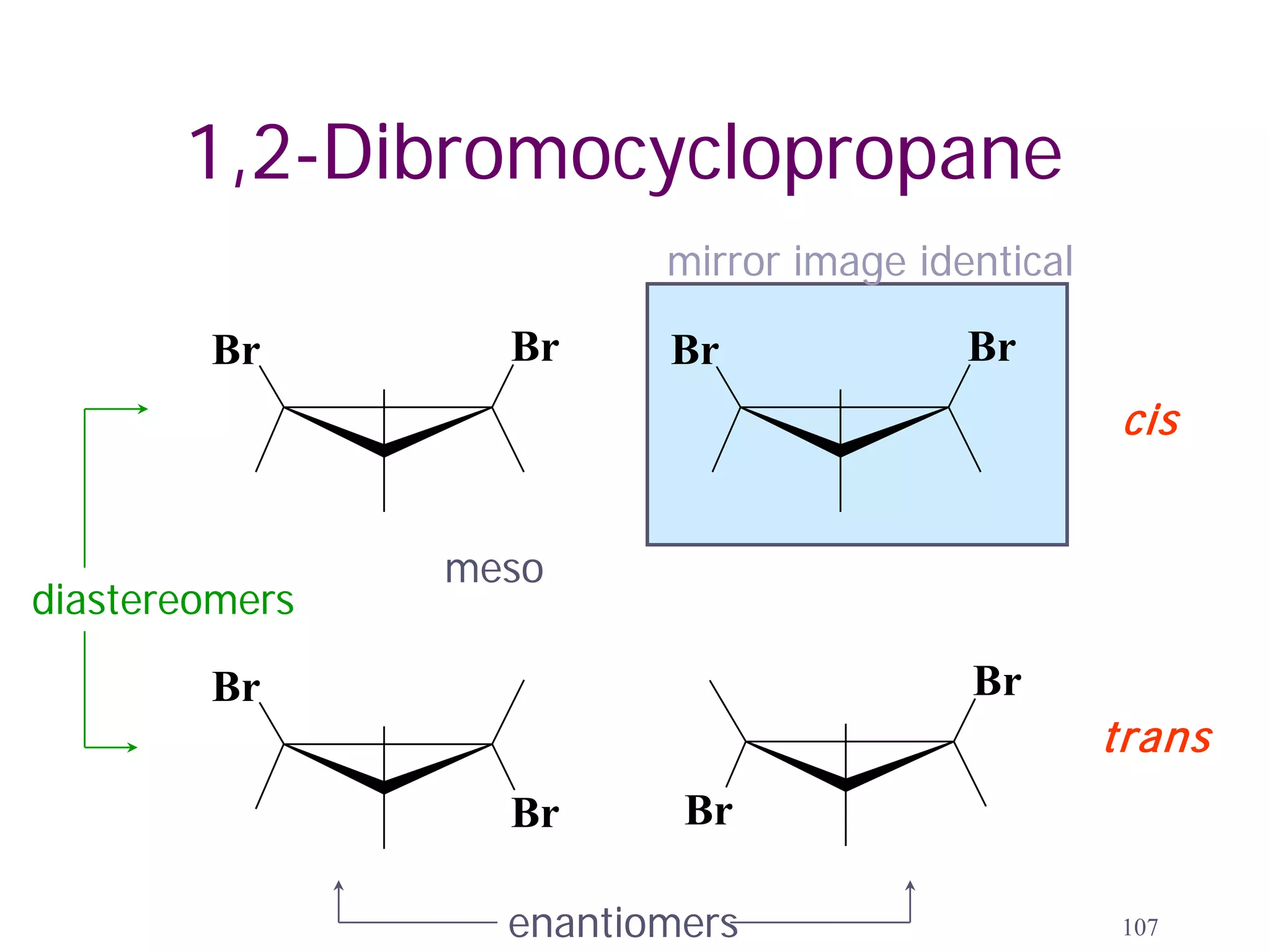
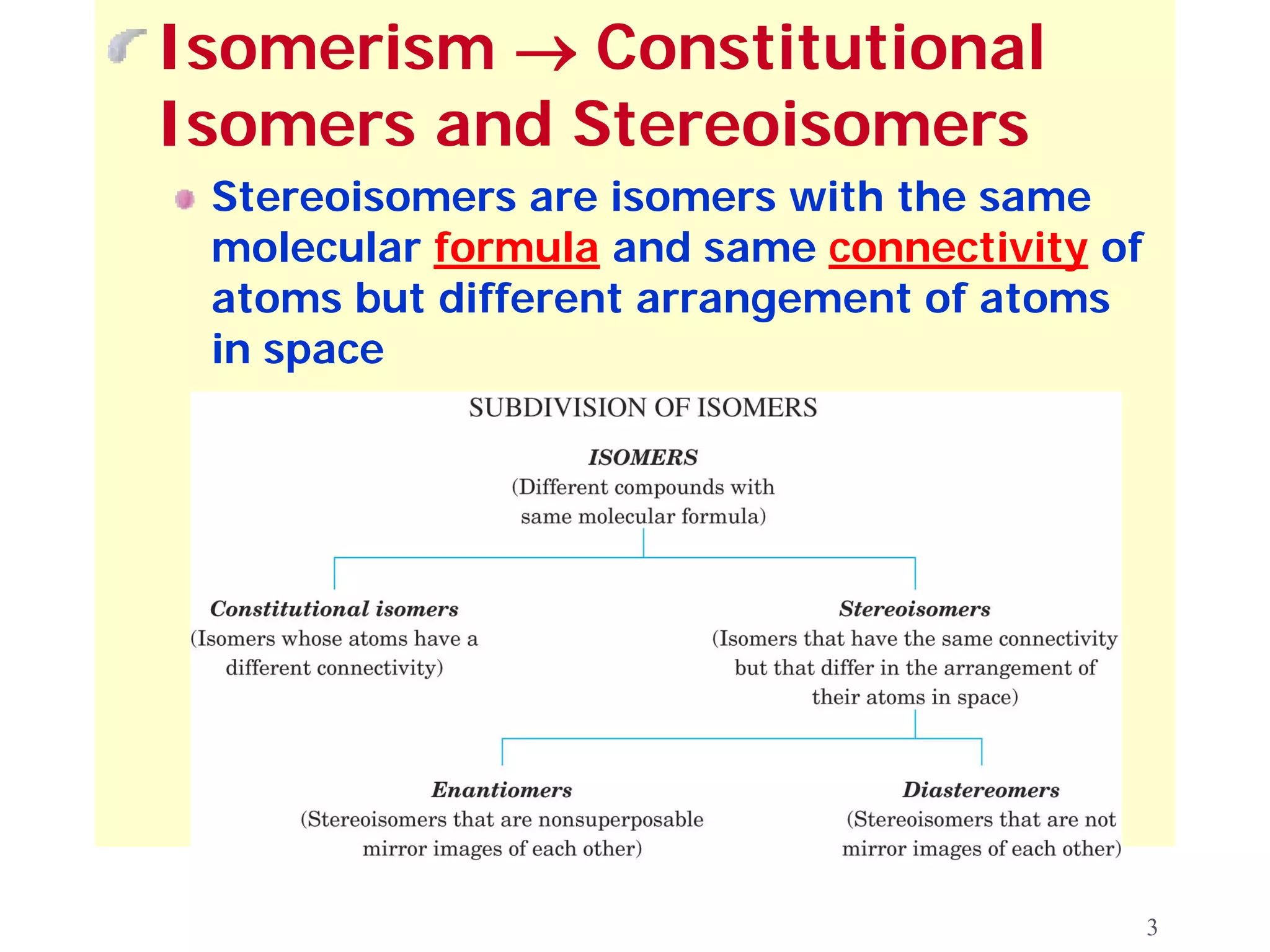
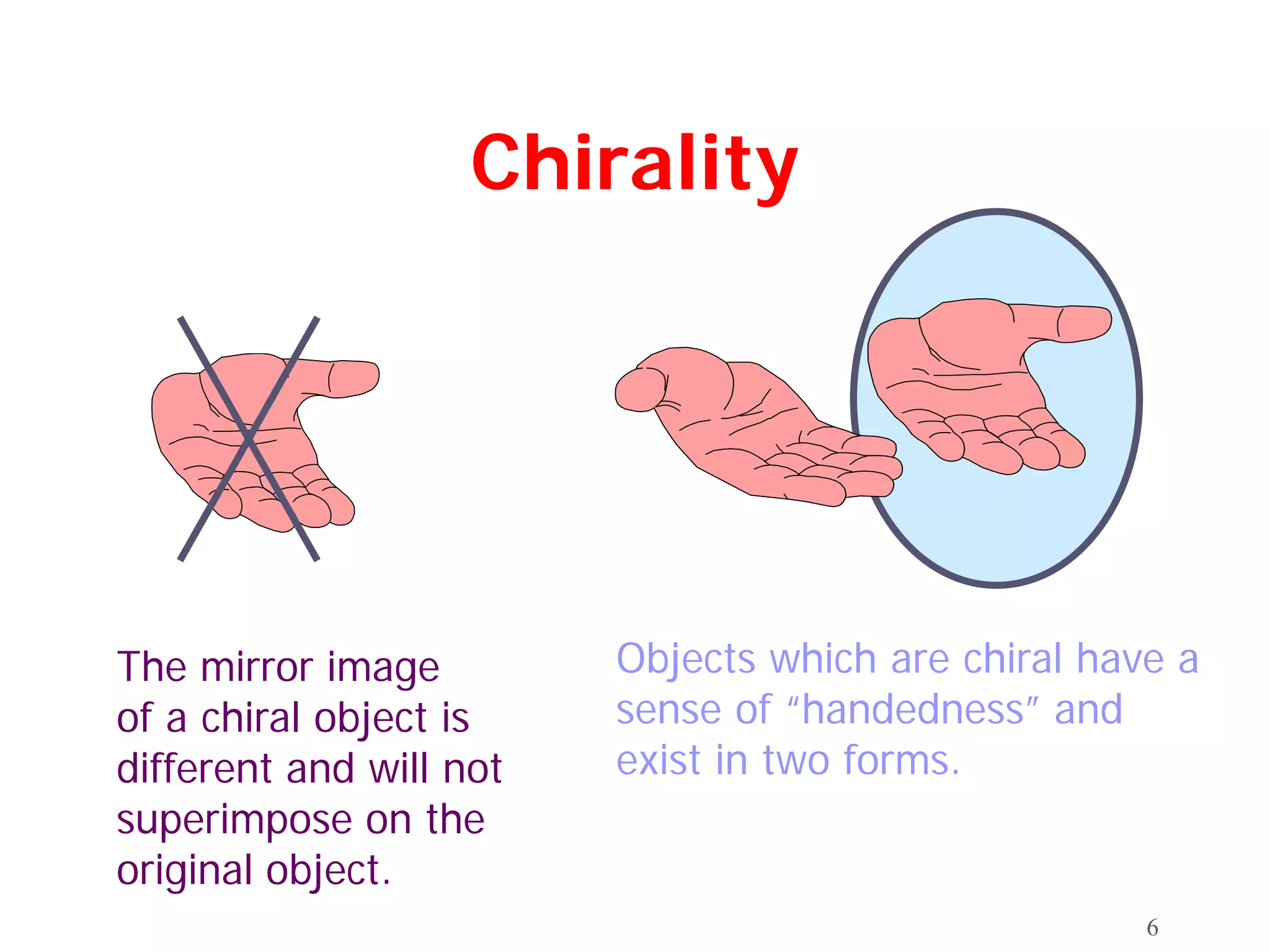
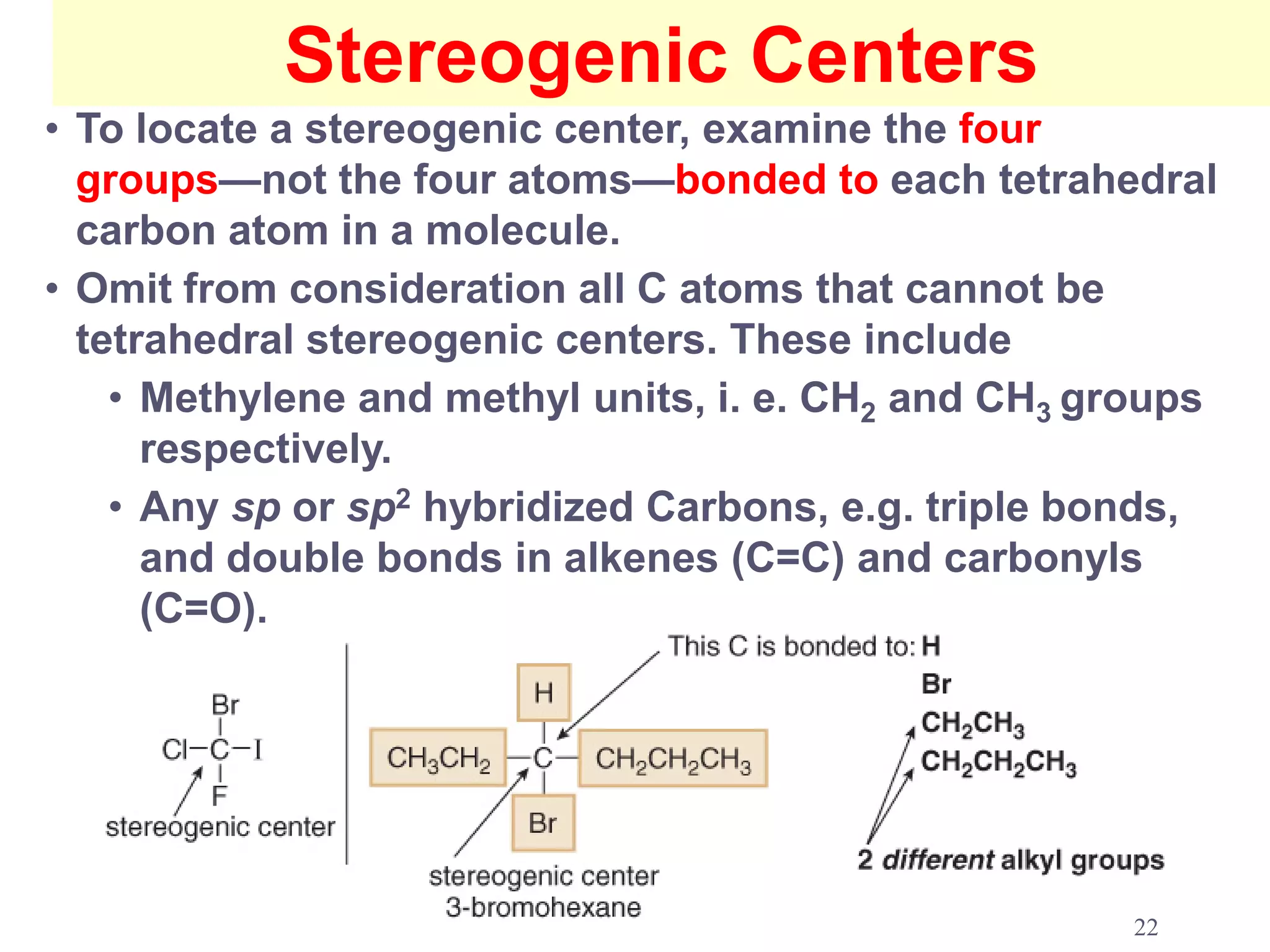
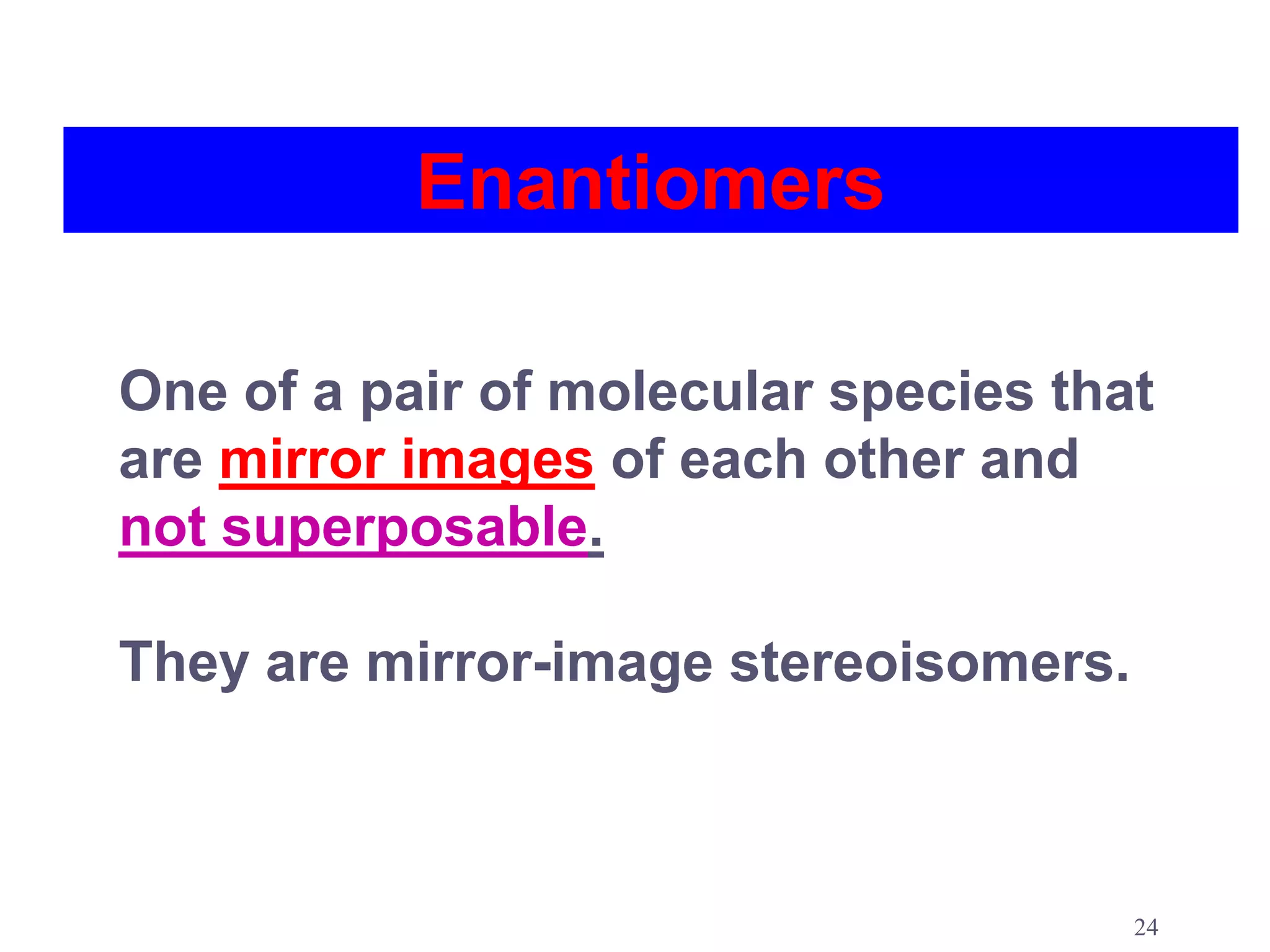
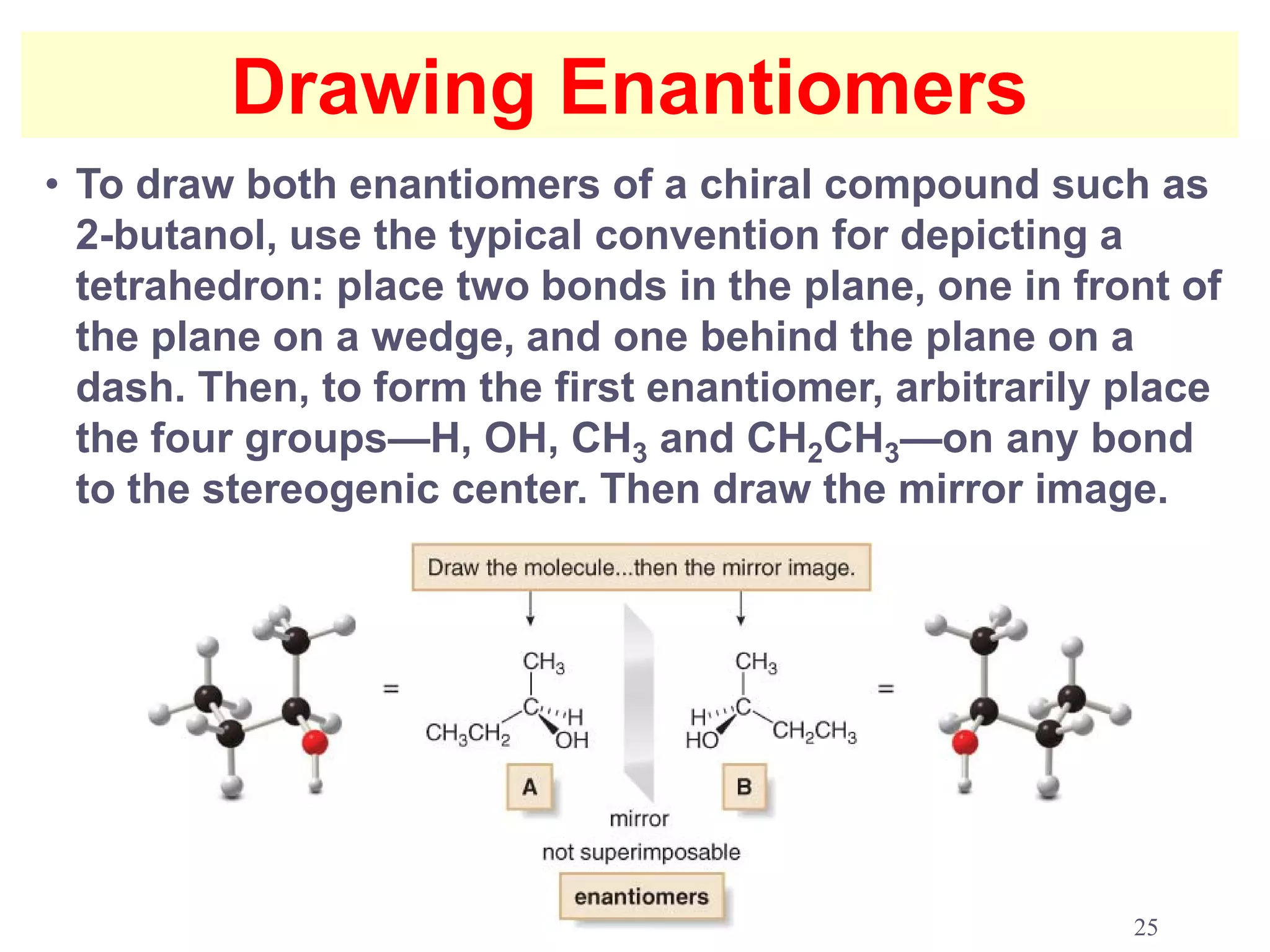
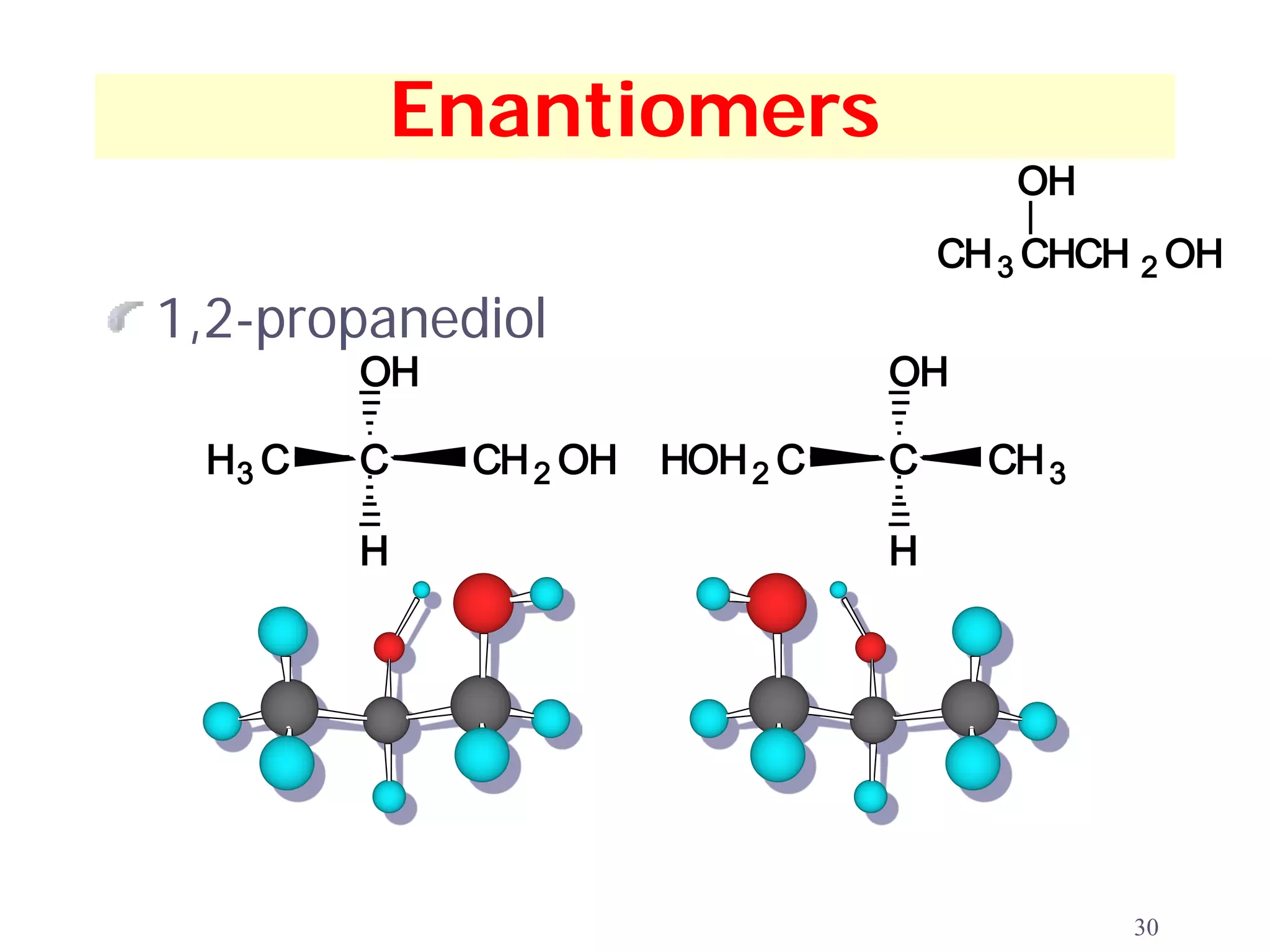
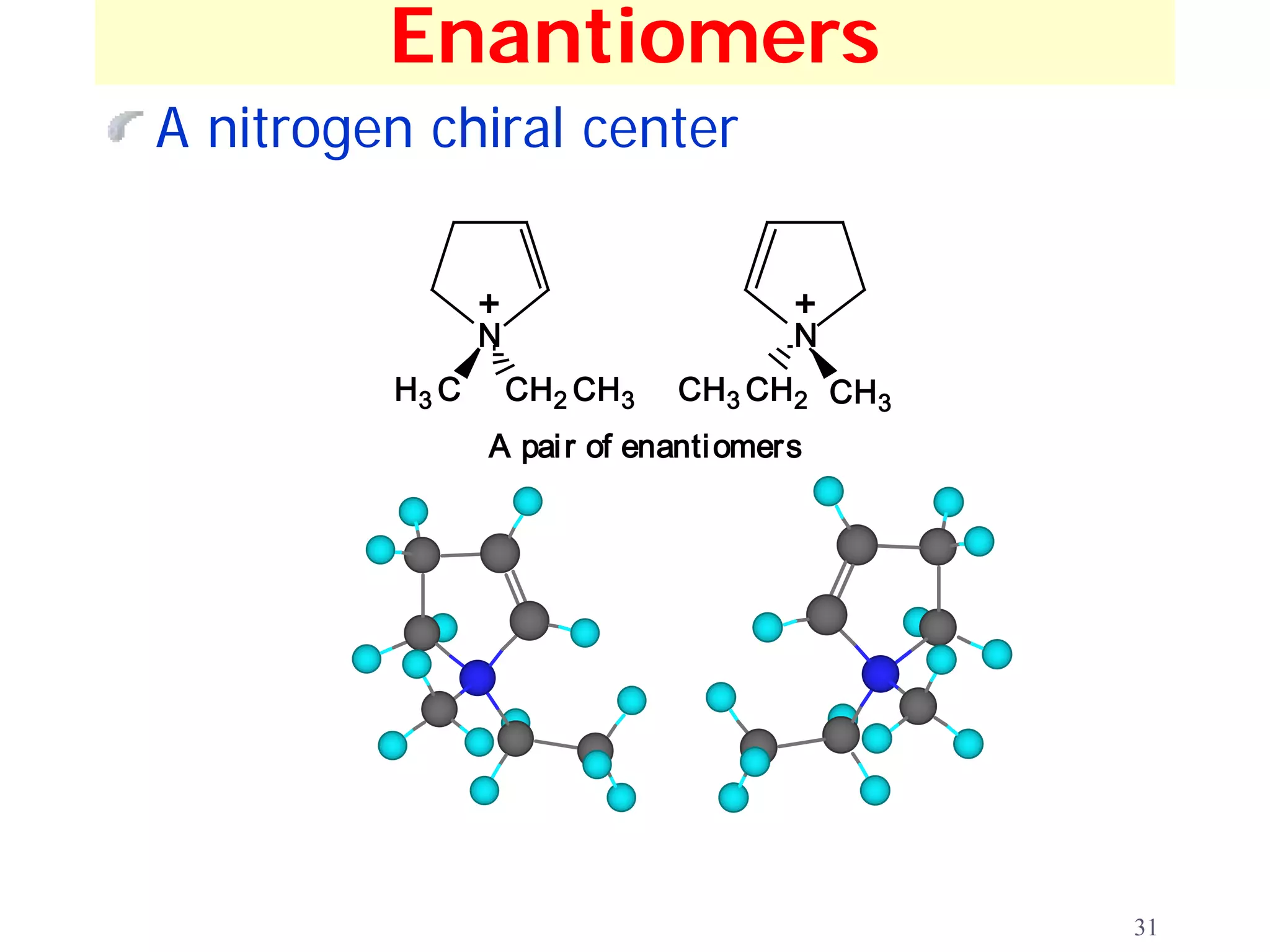
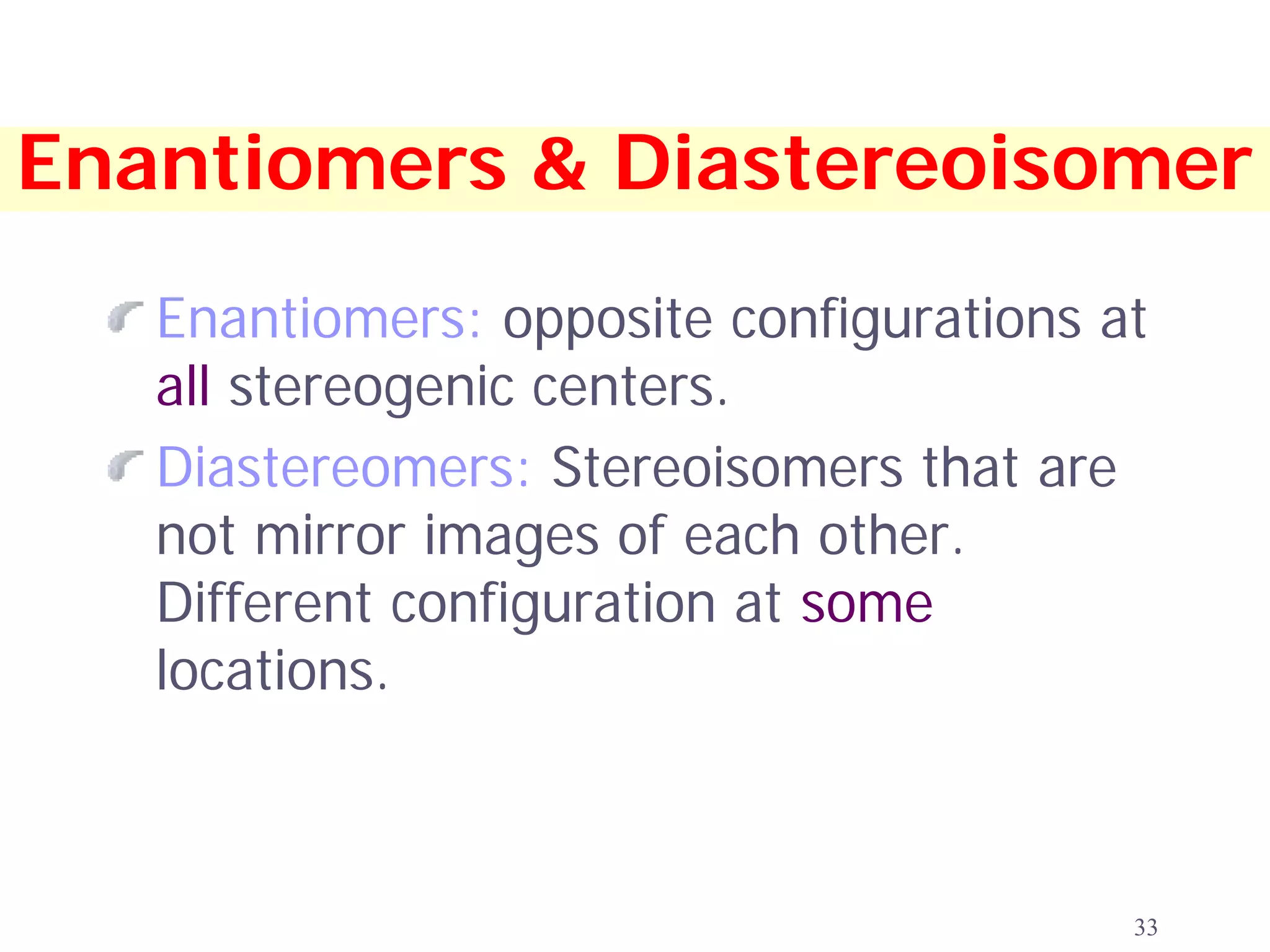
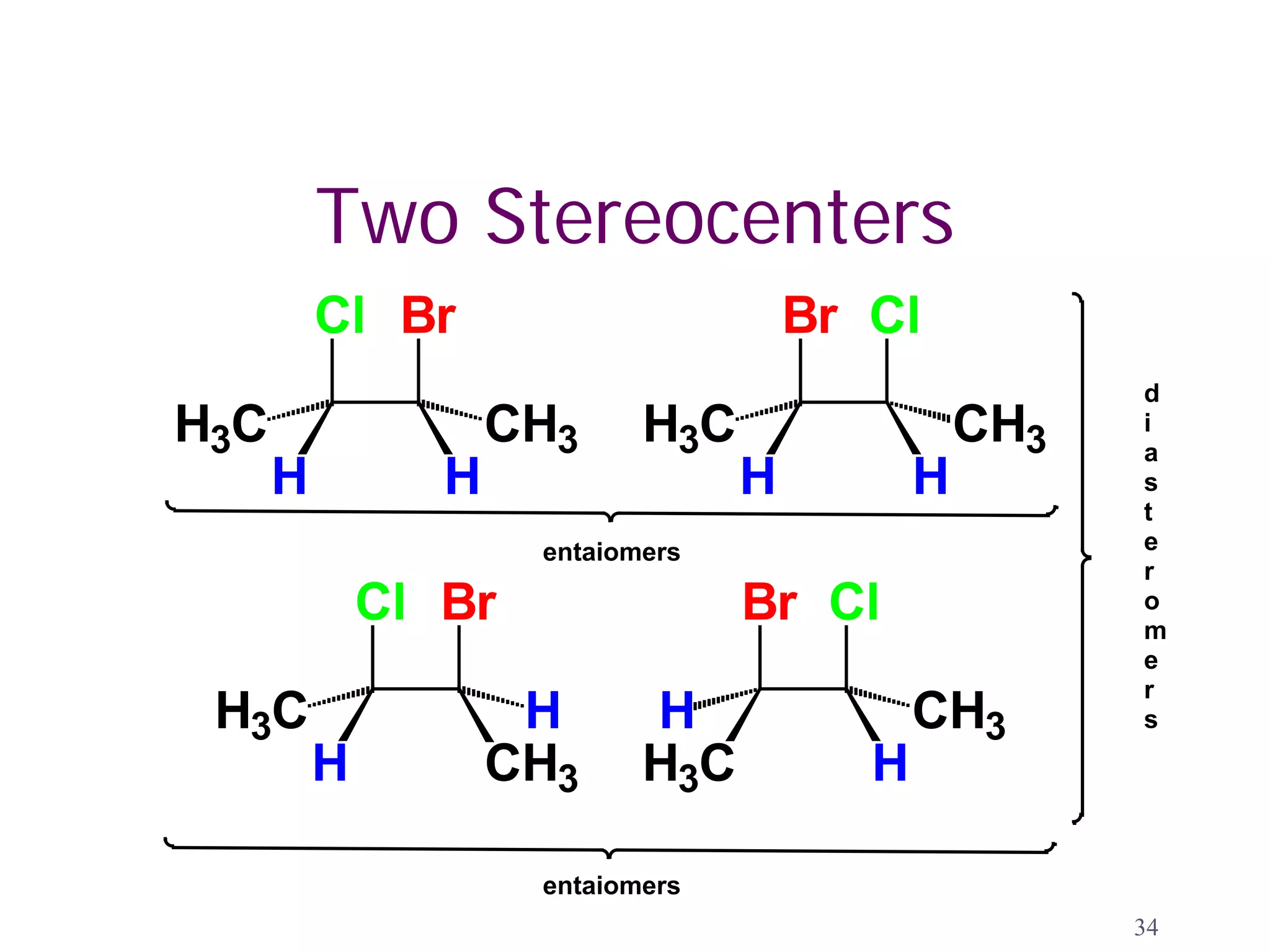
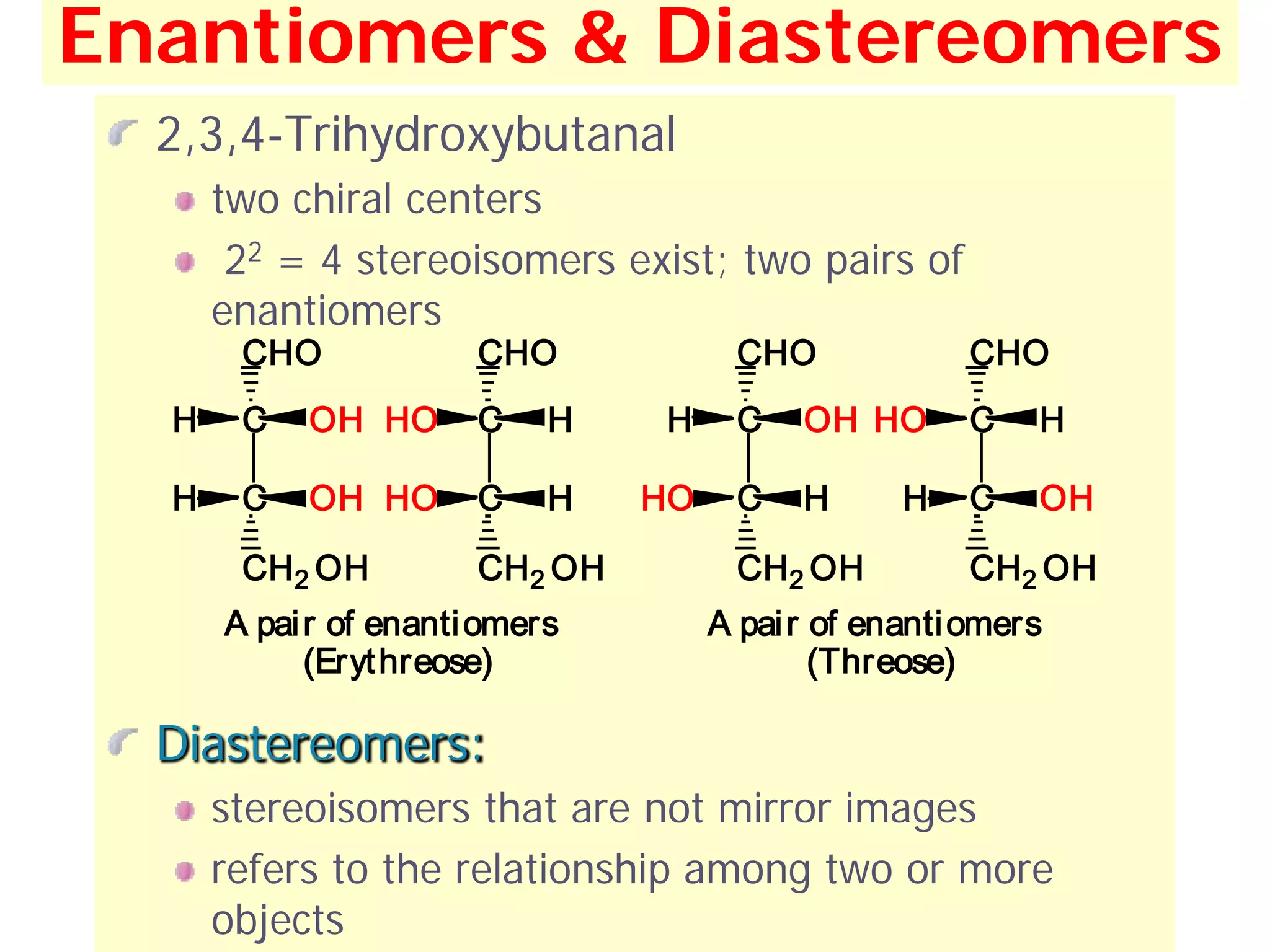
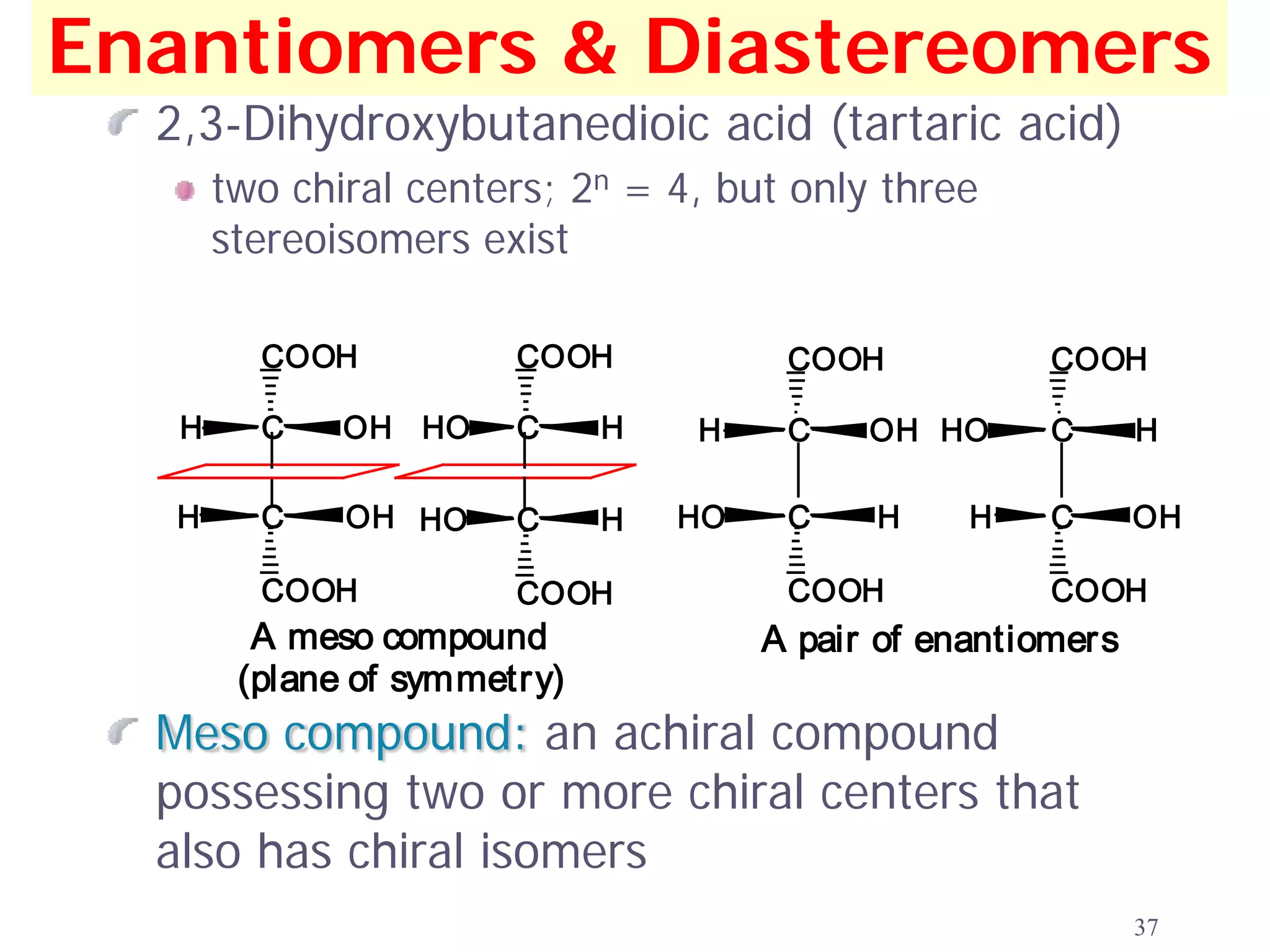
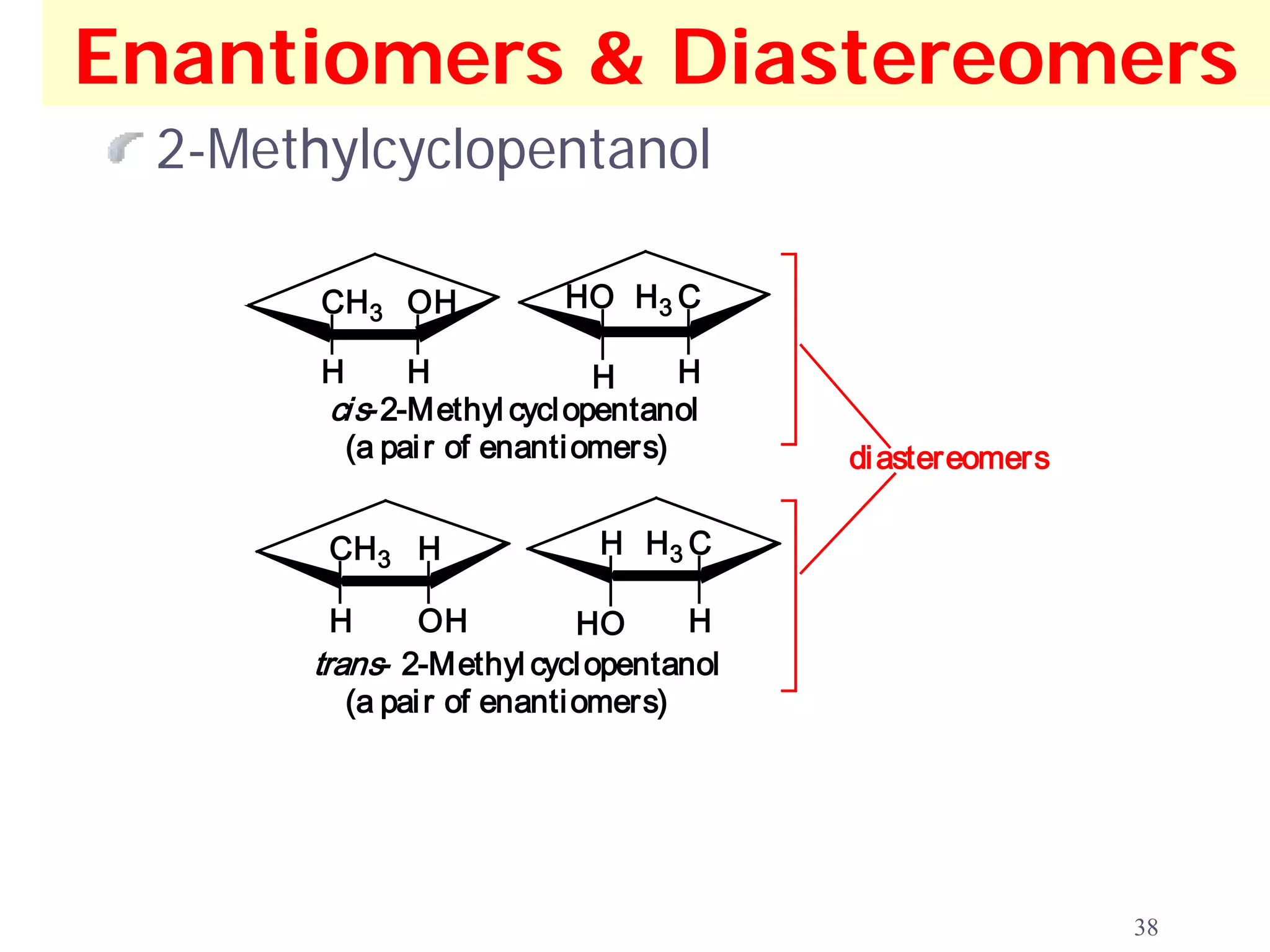
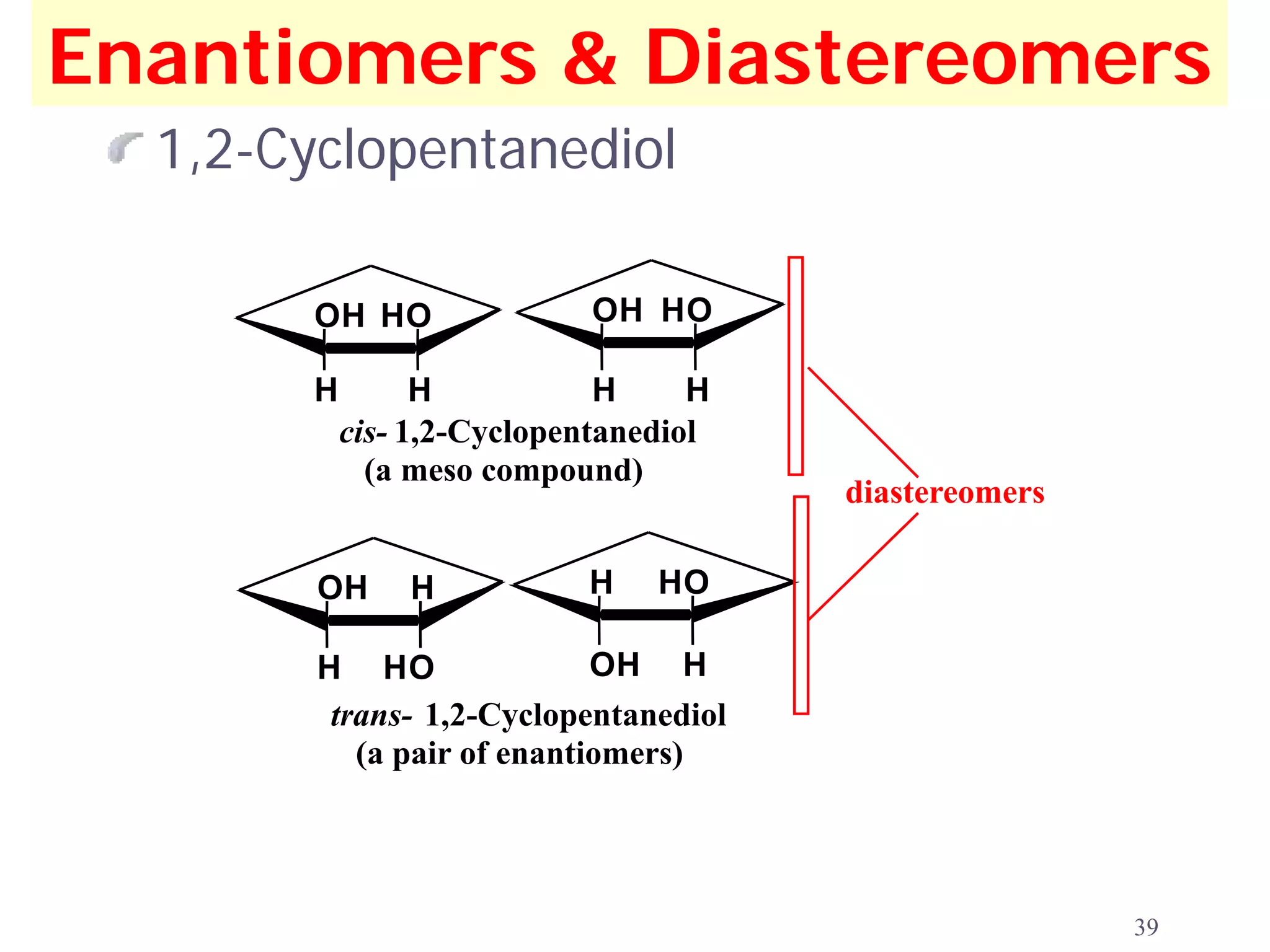
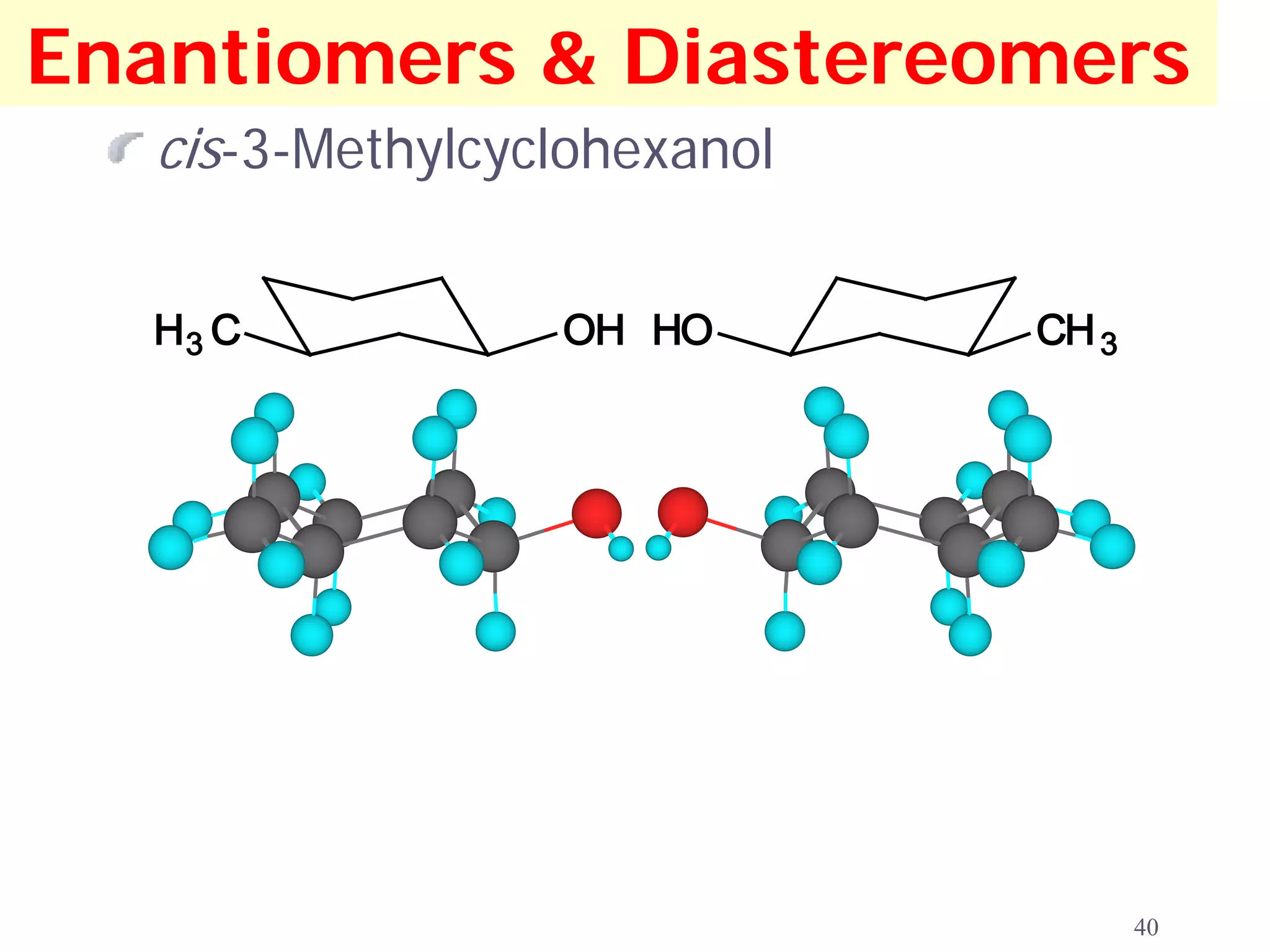
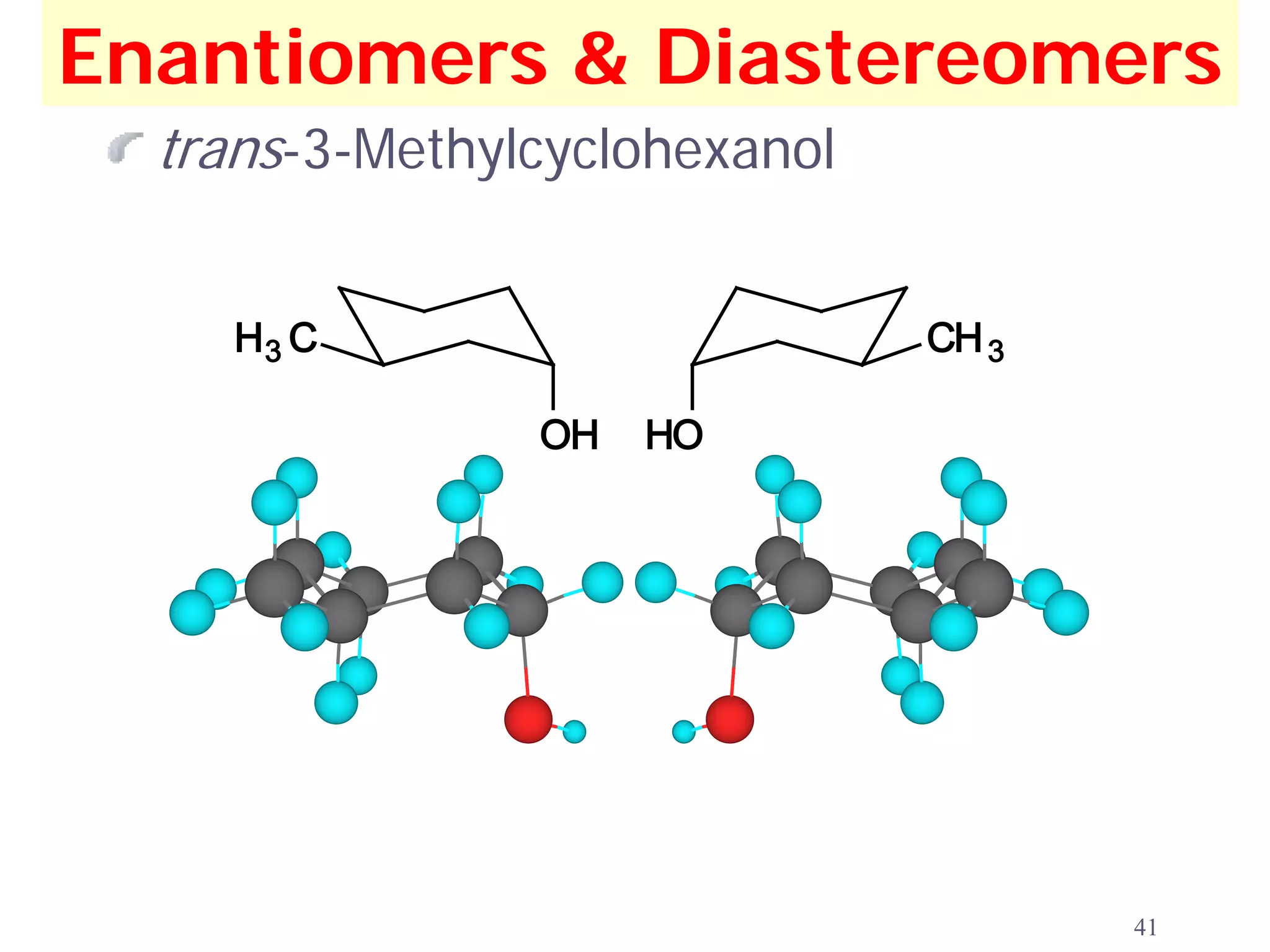
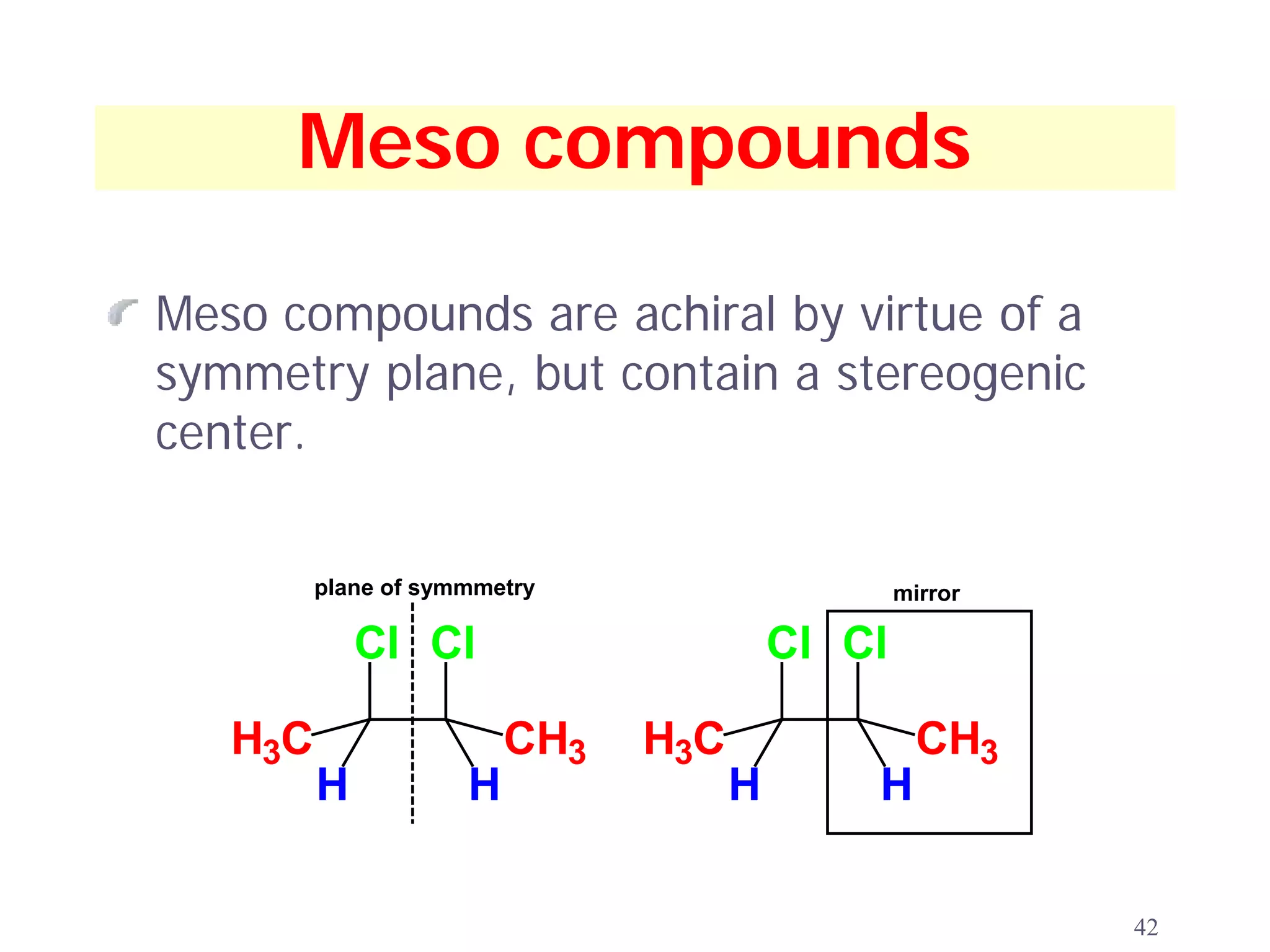
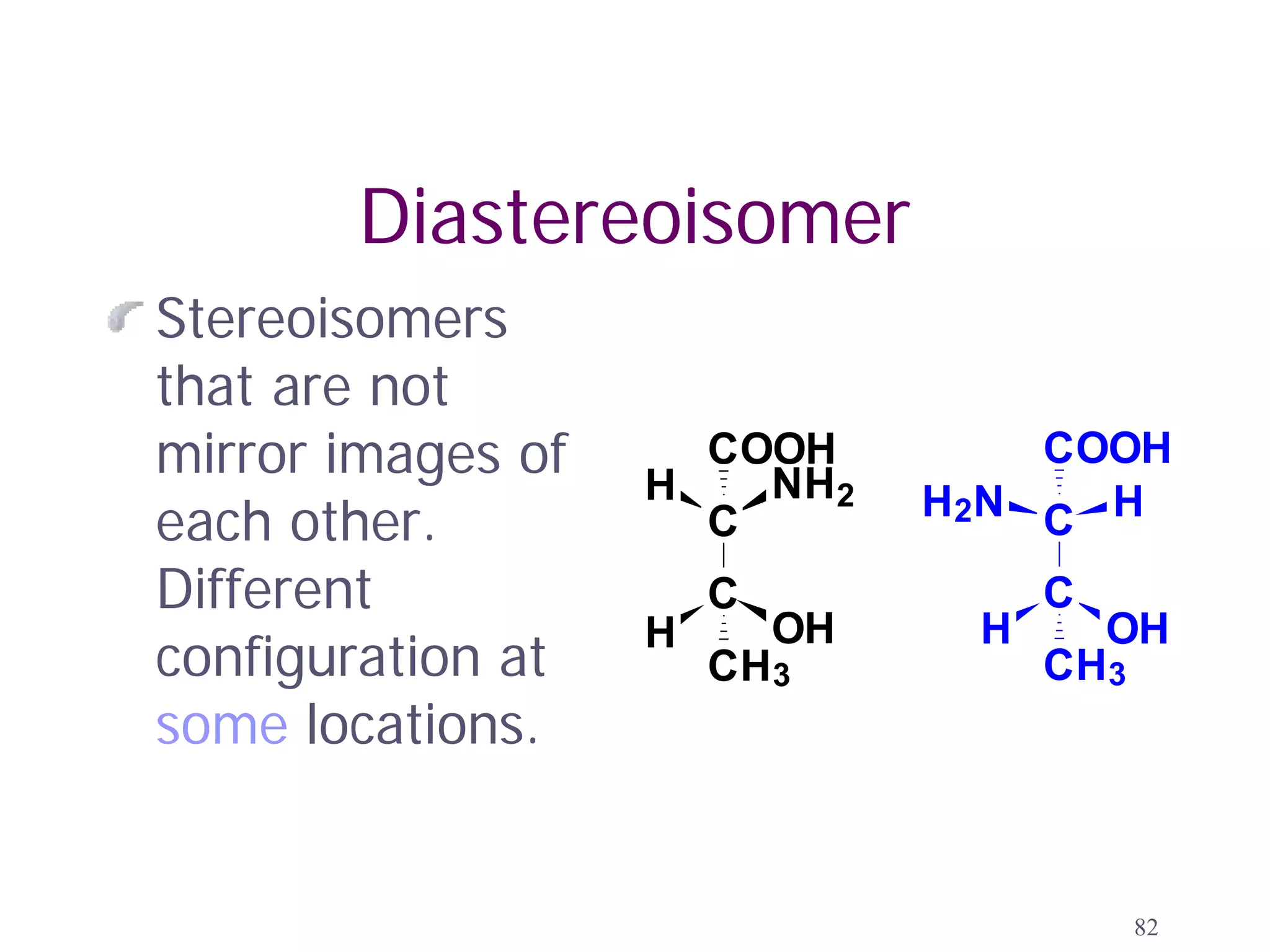
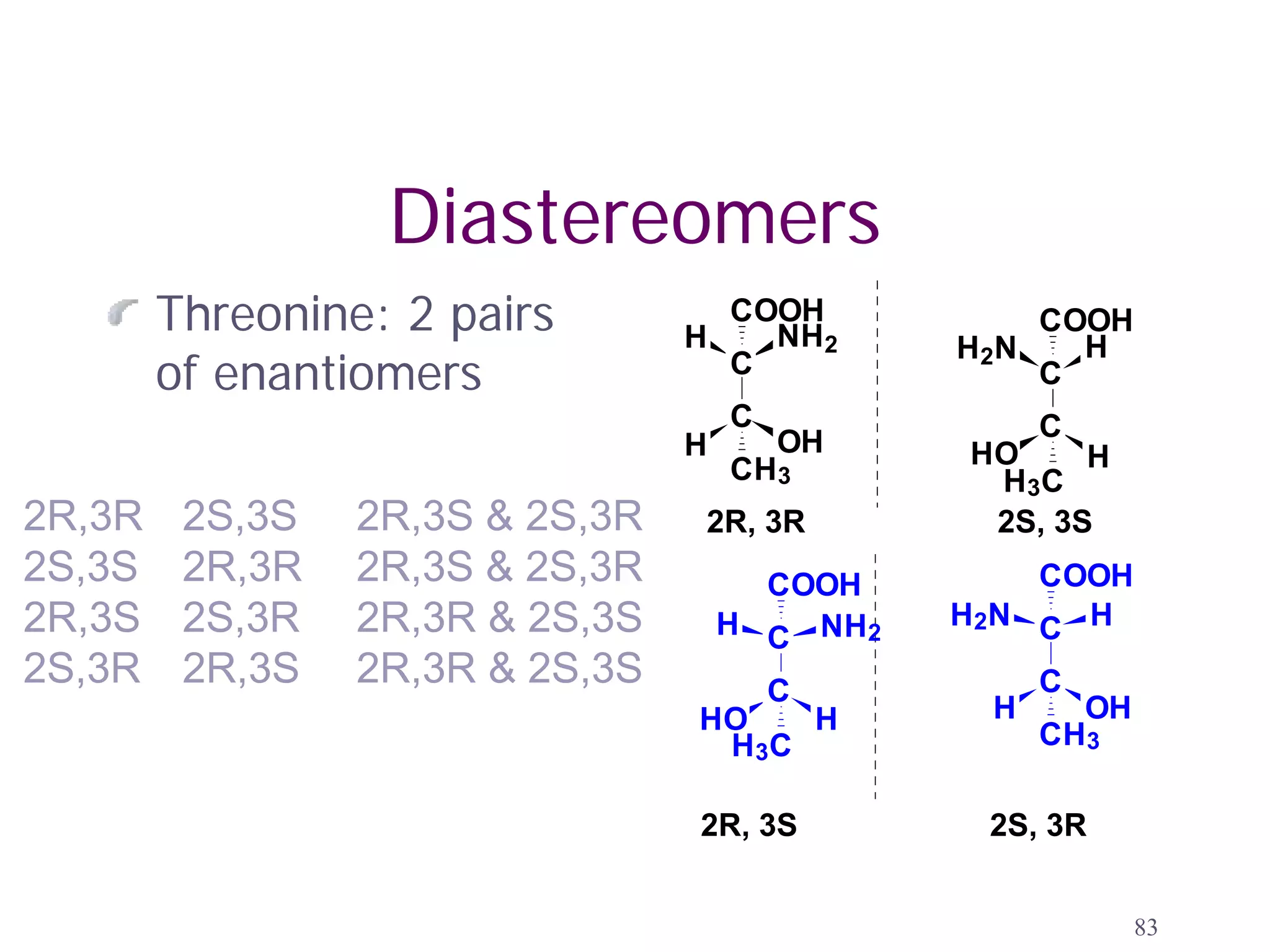
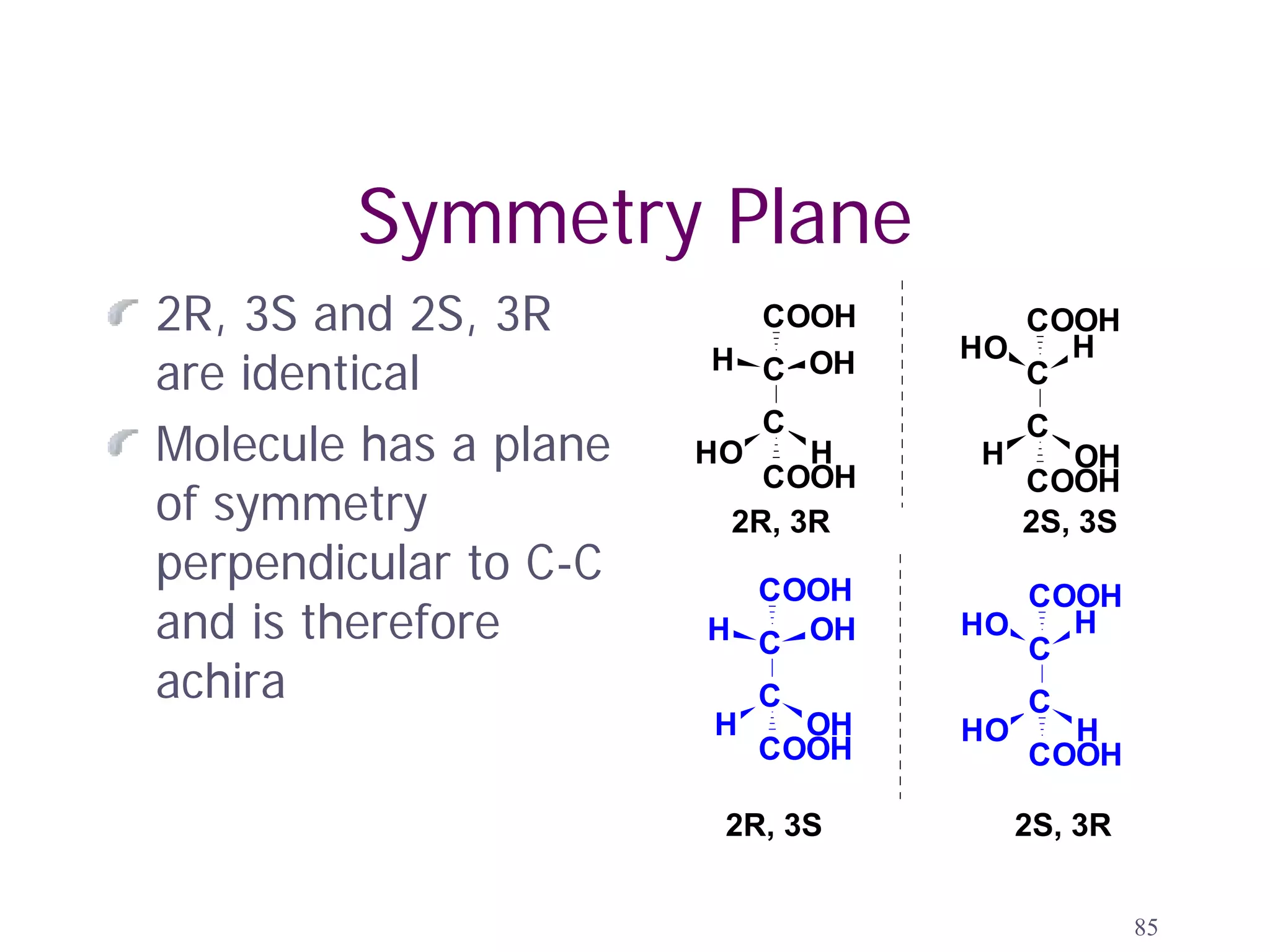
2. Key terms defined include constitutional isomers, stereoisomers, chiral molecules, stereogenic centers, enantiomers, and diastereomers.
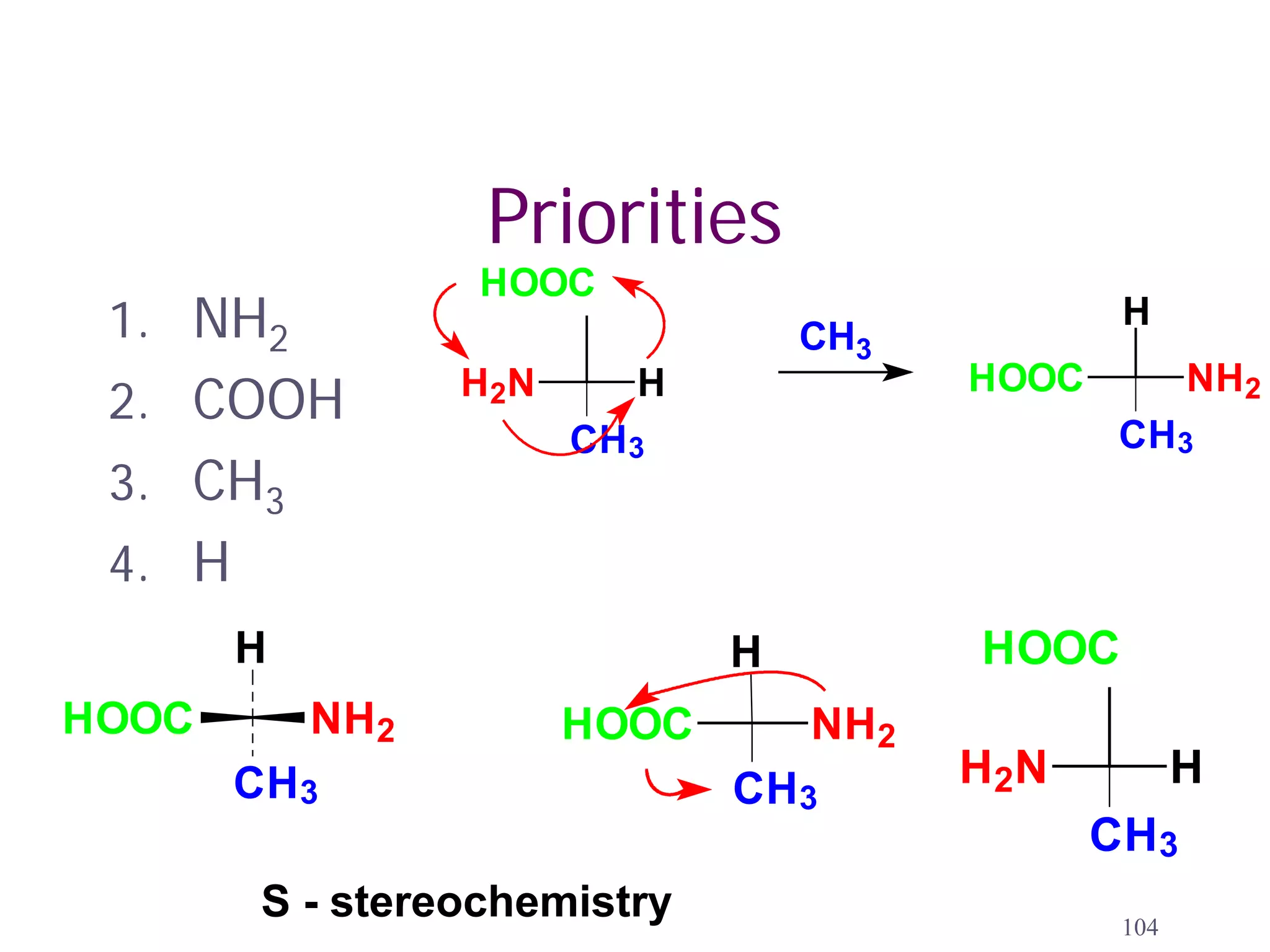
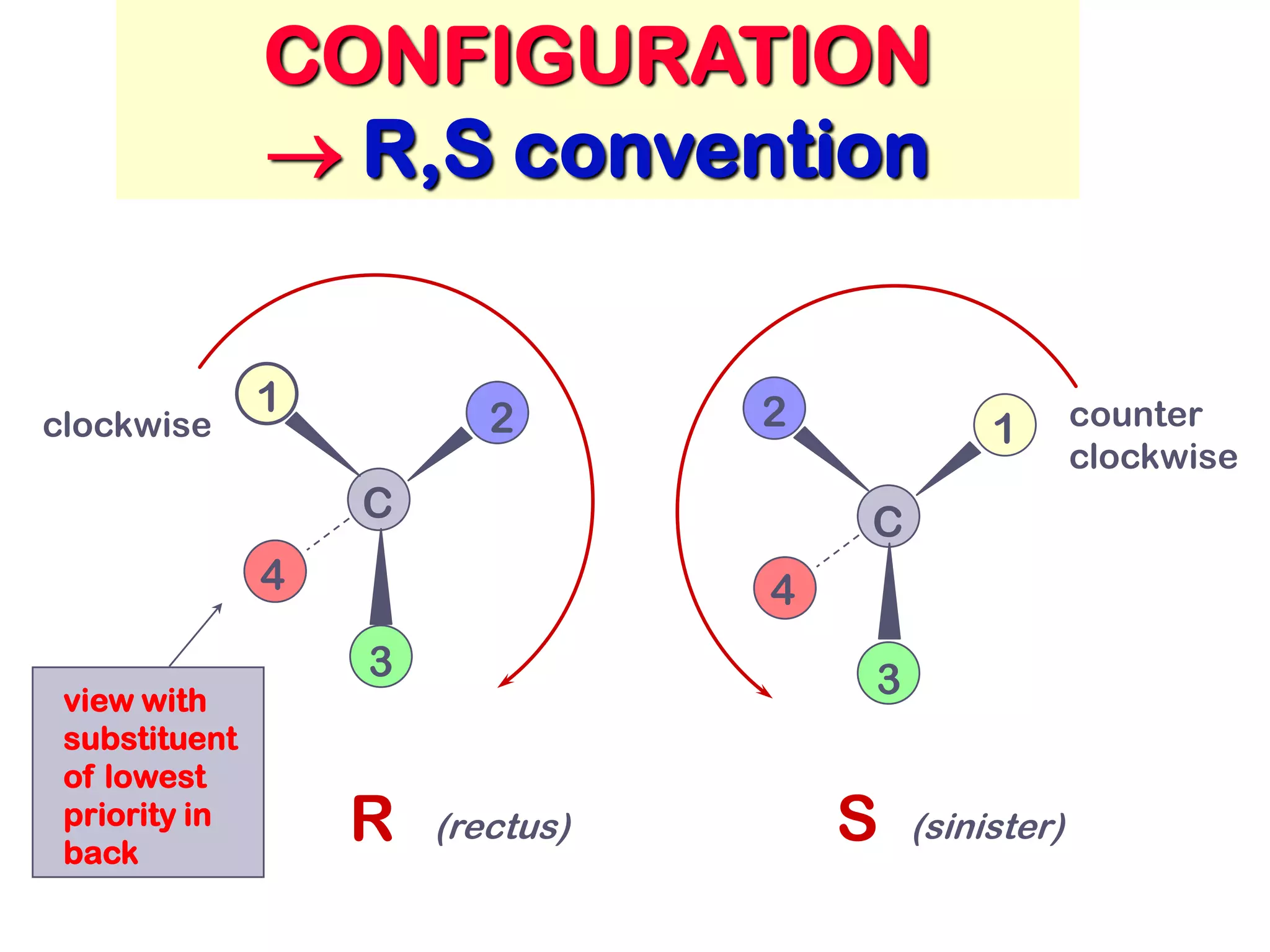
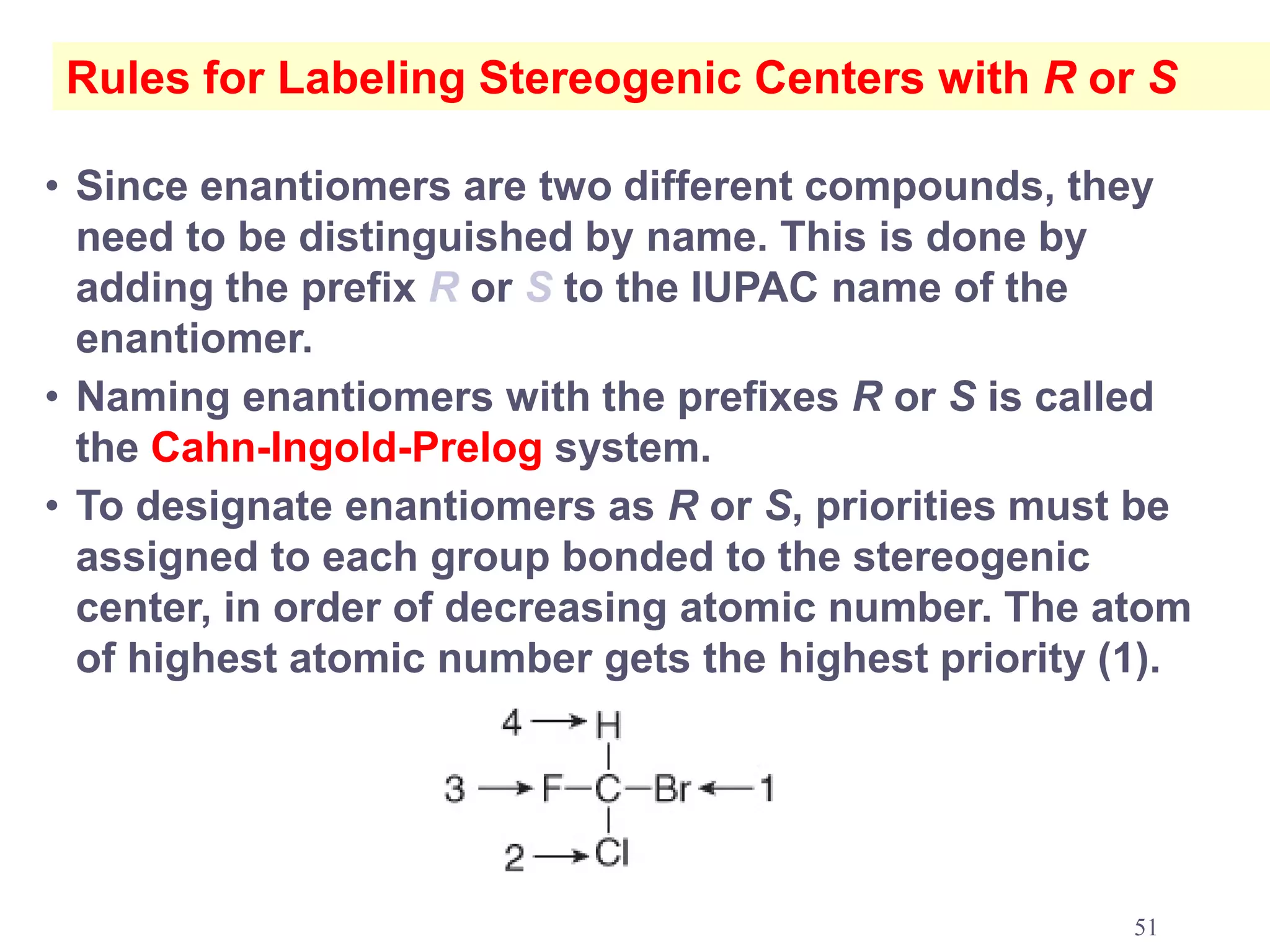
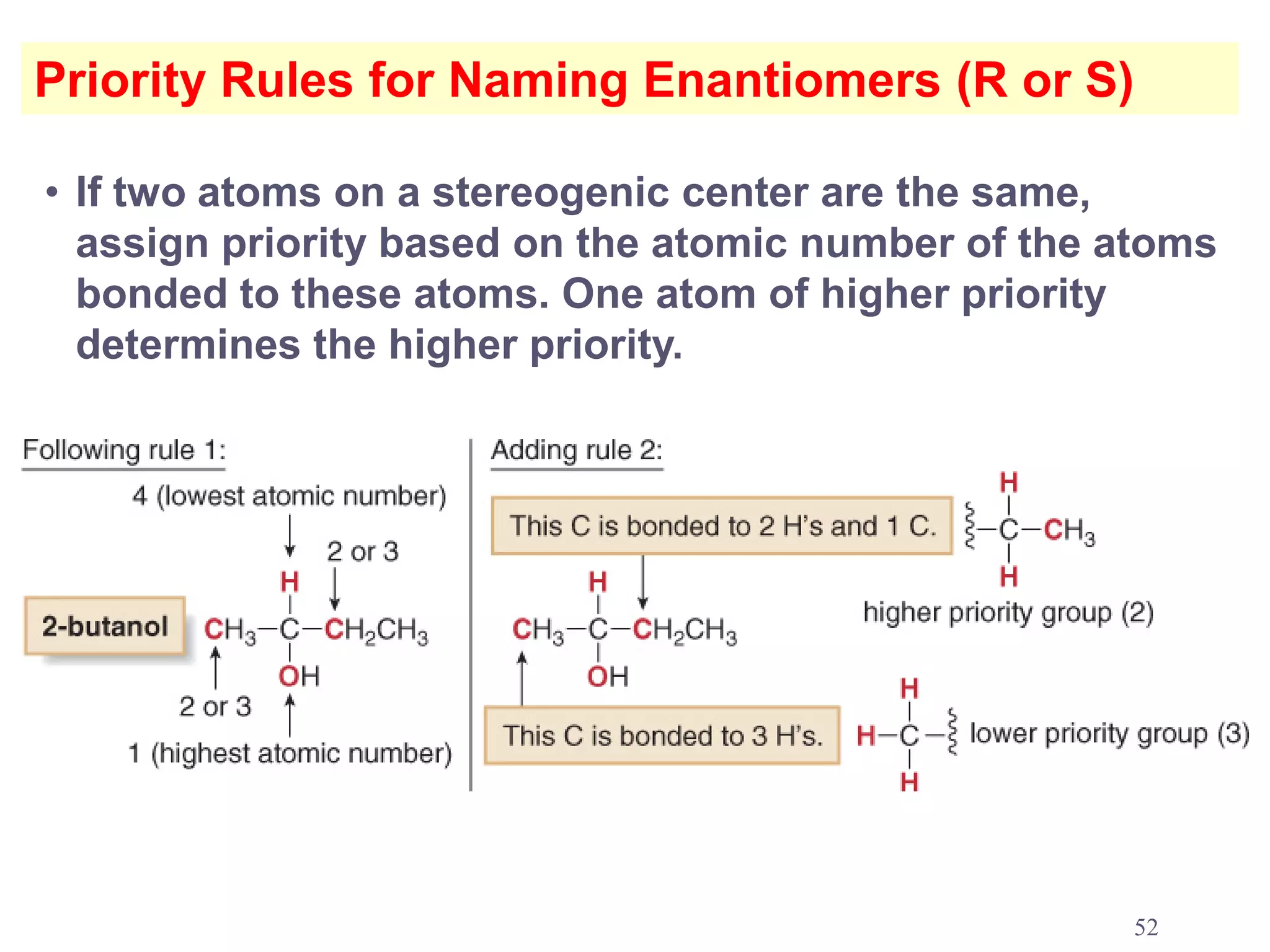
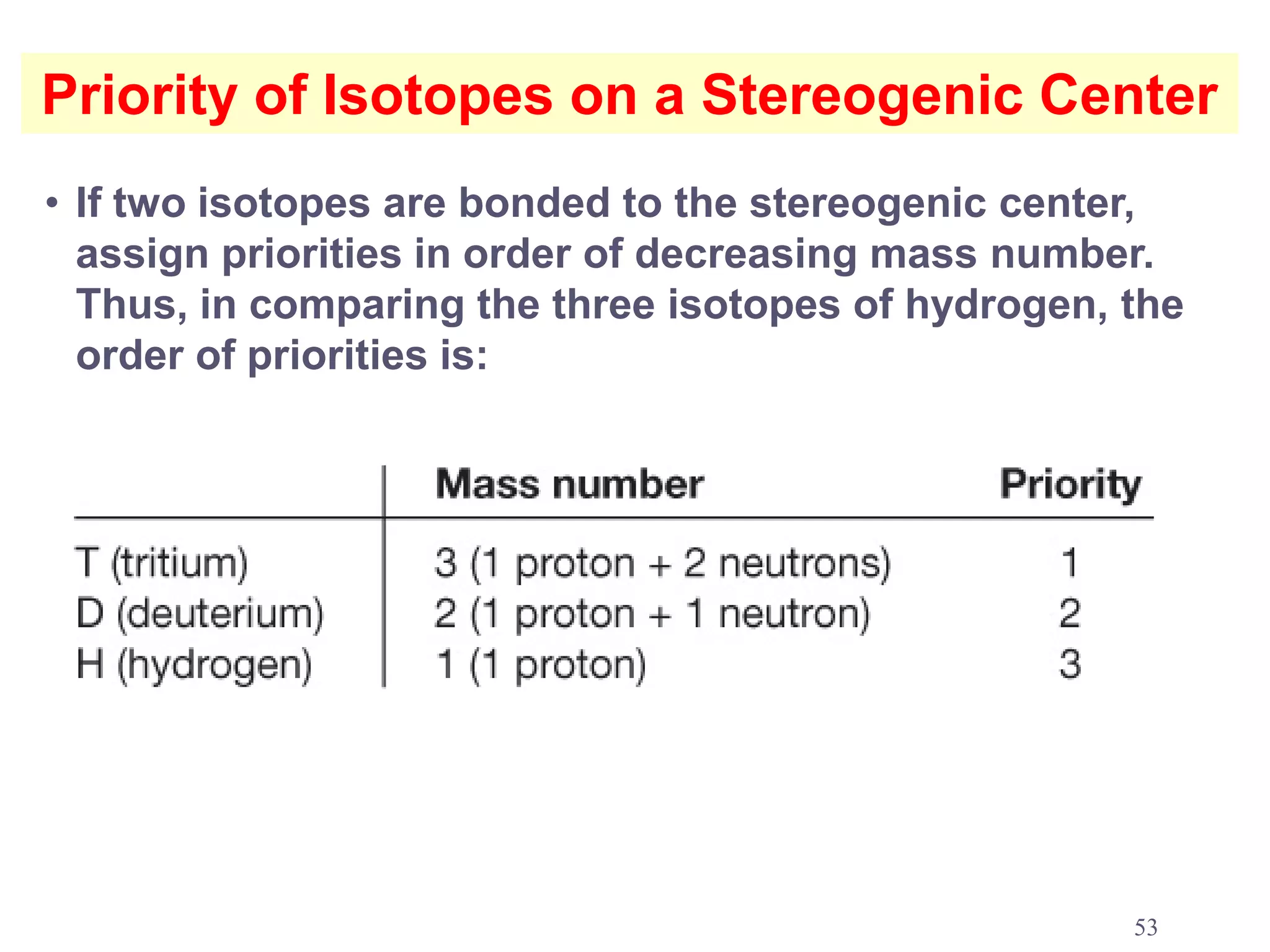
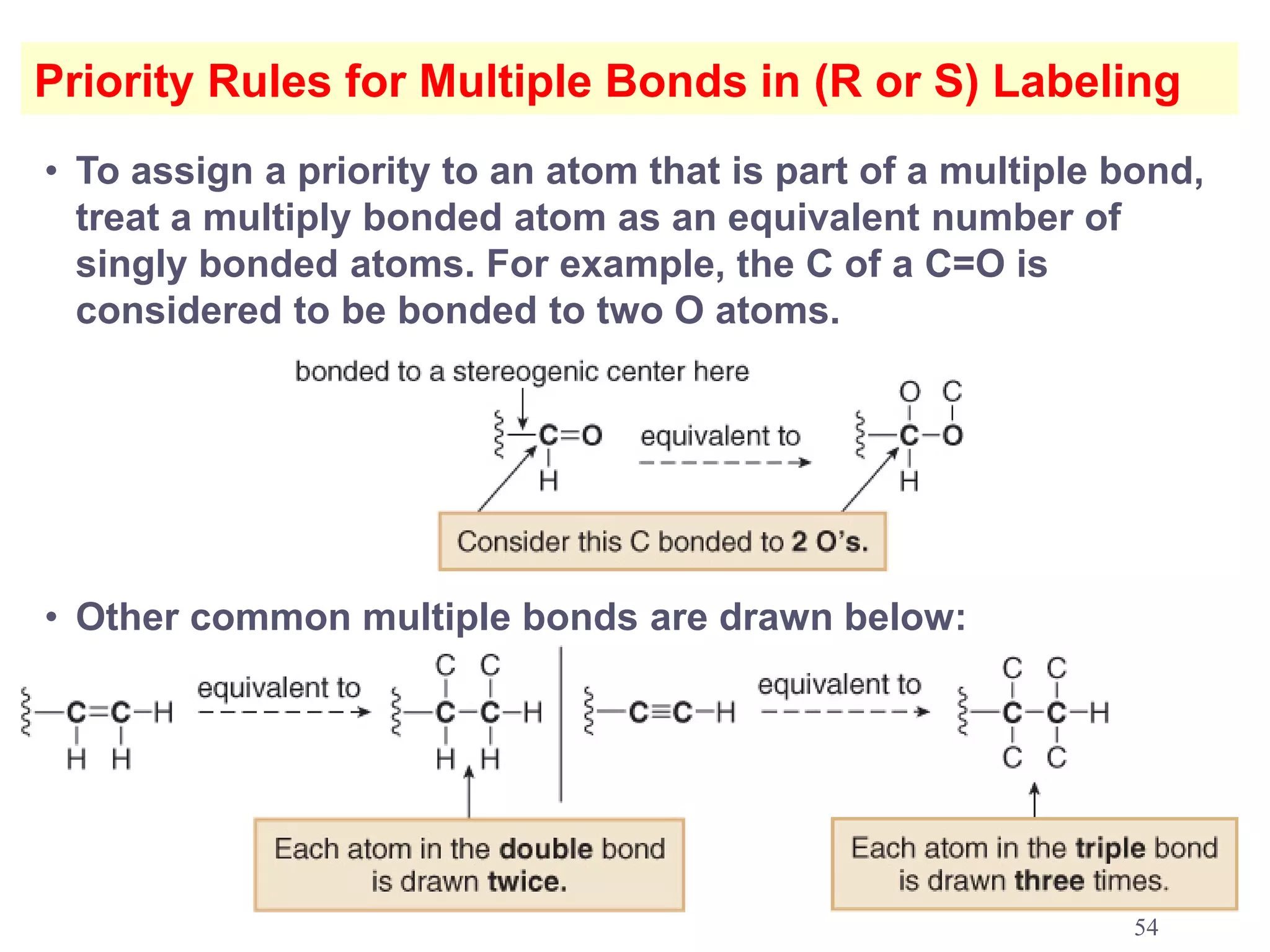
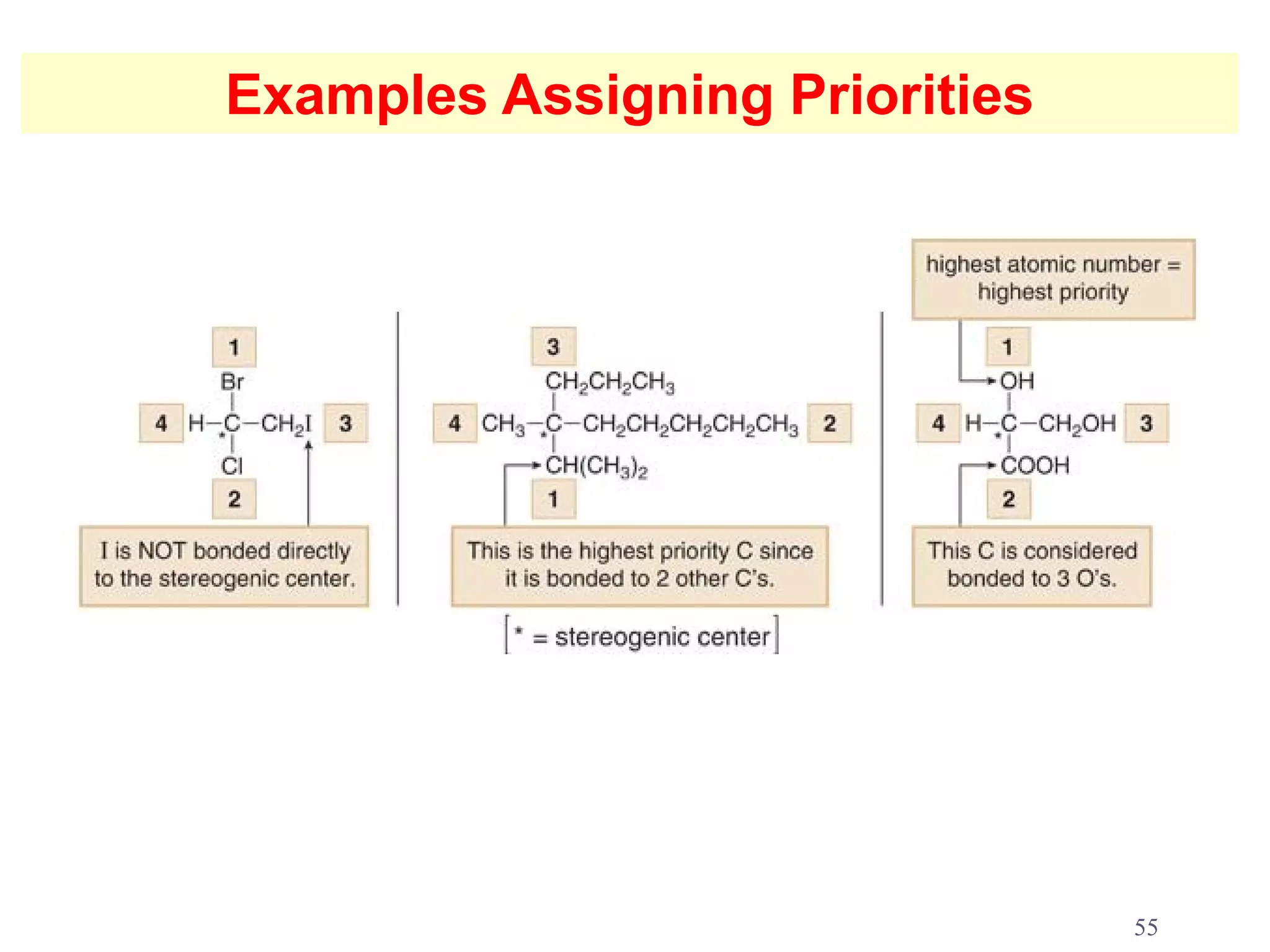
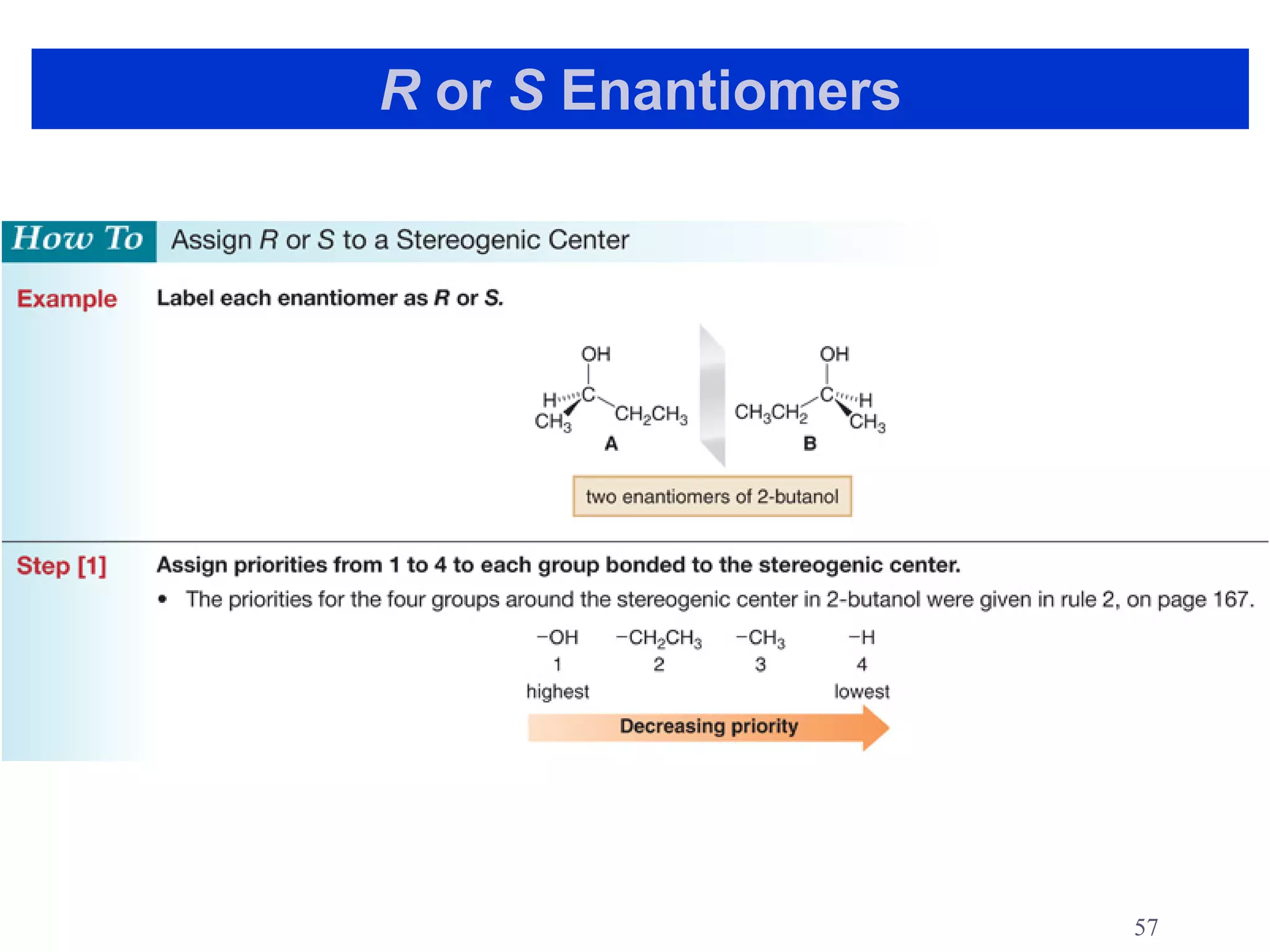
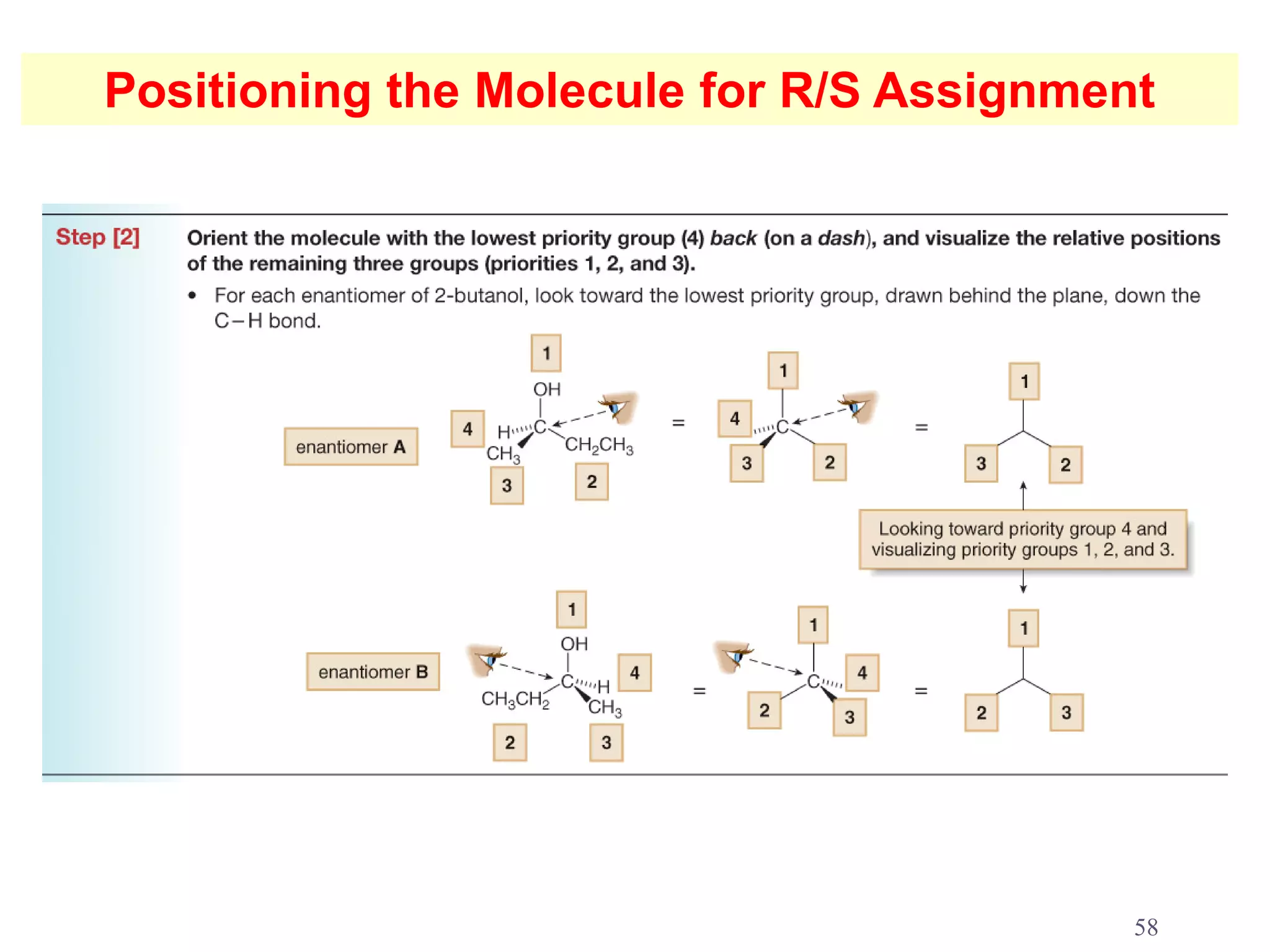
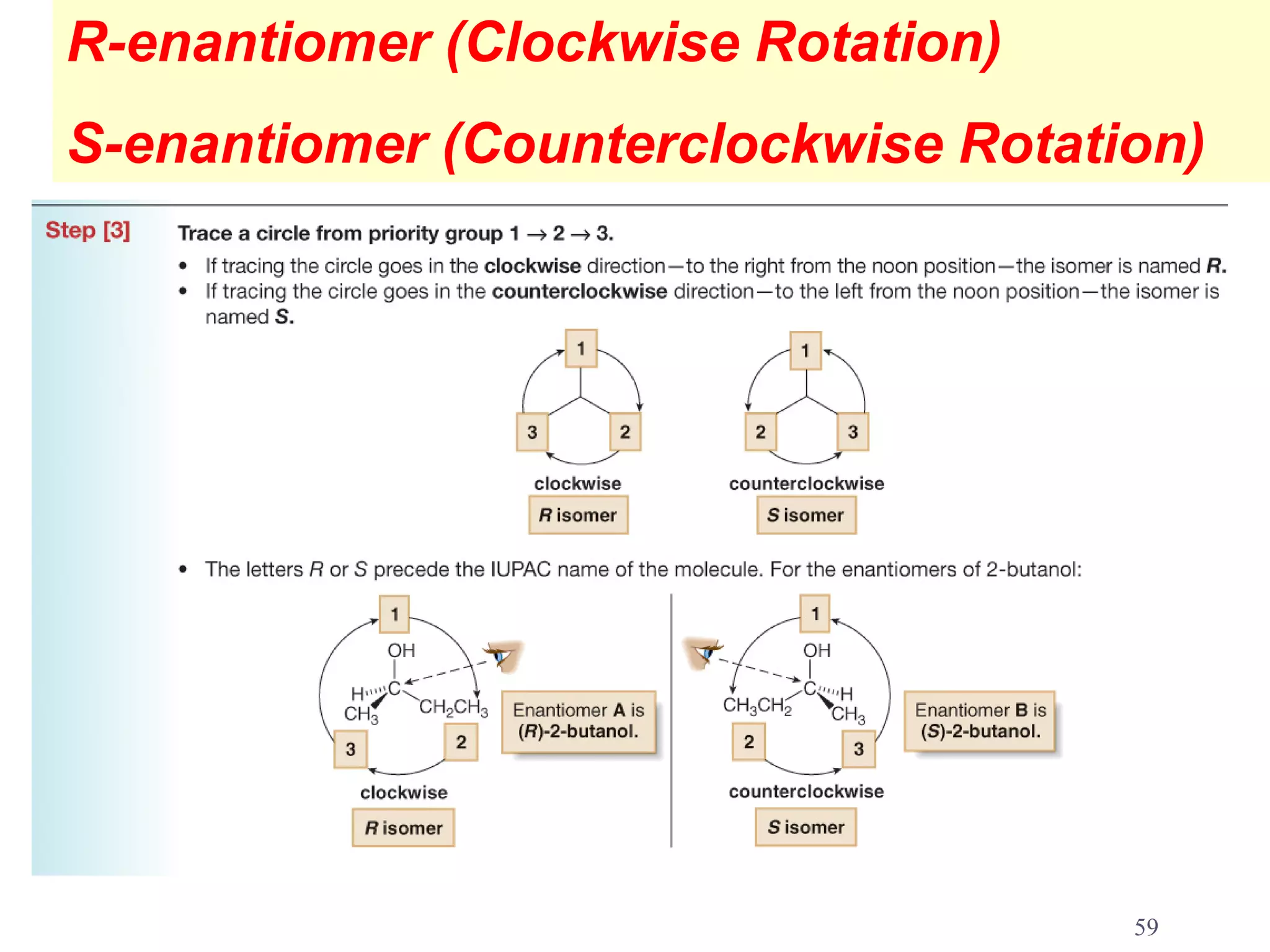
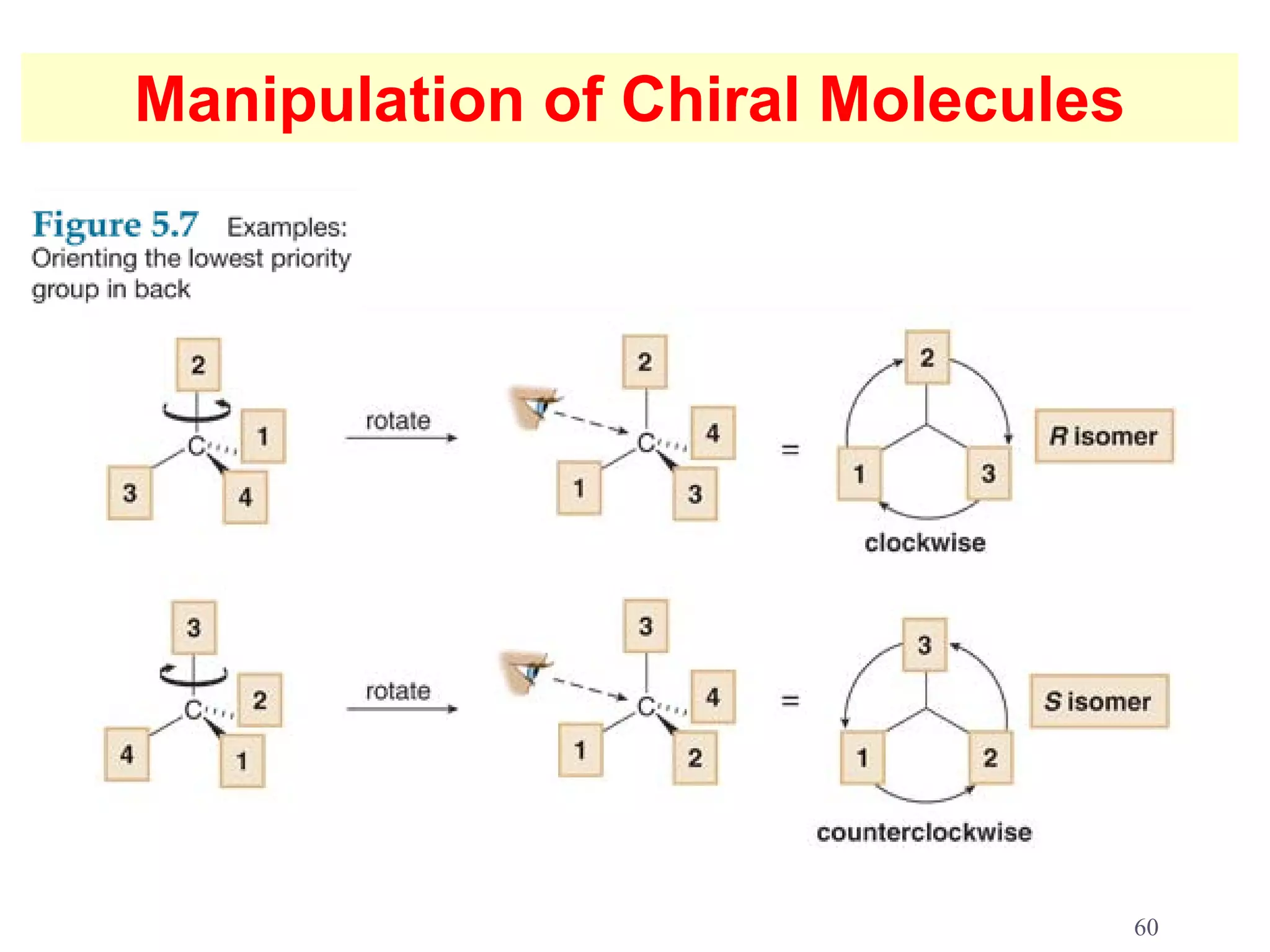
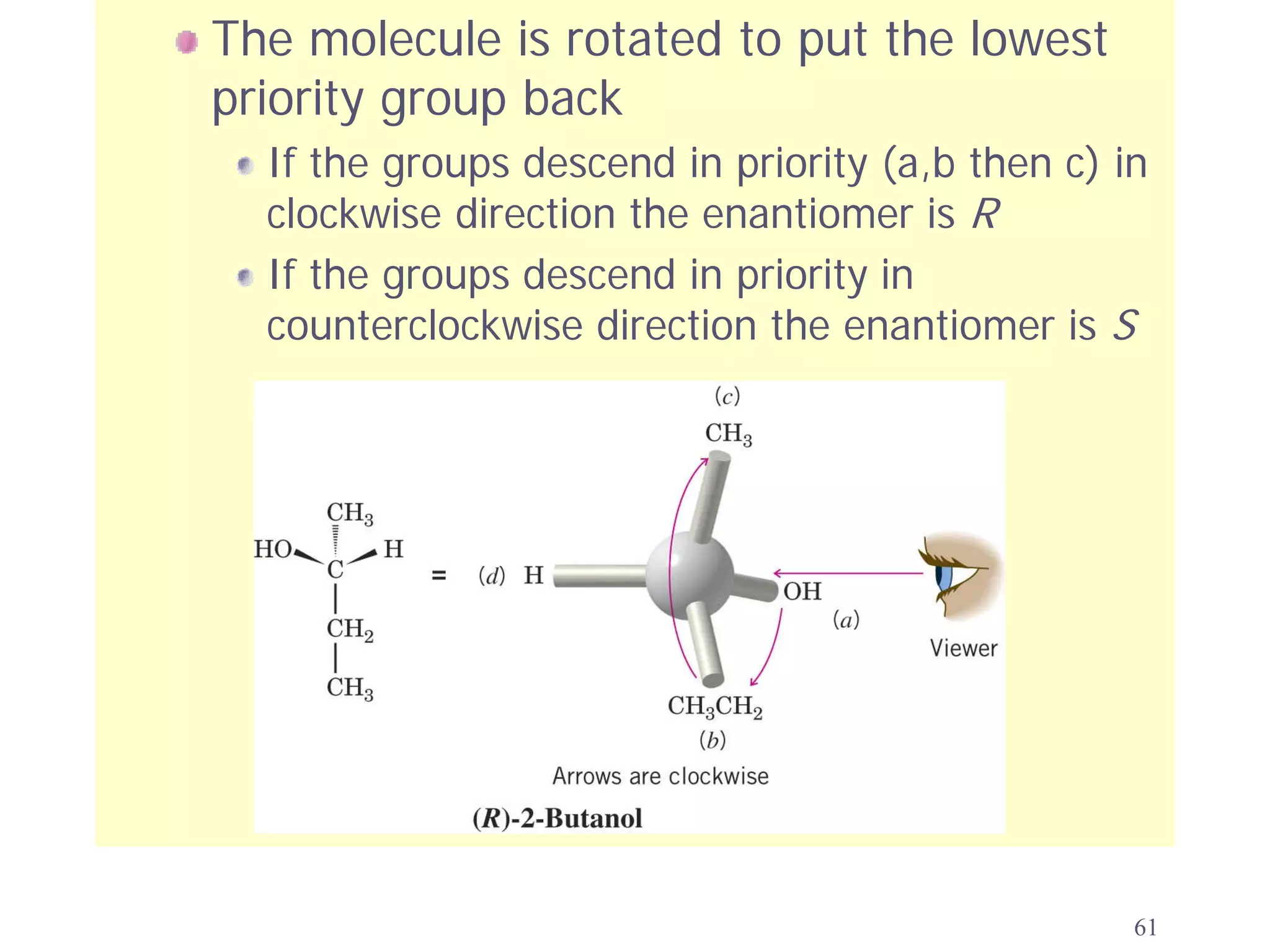
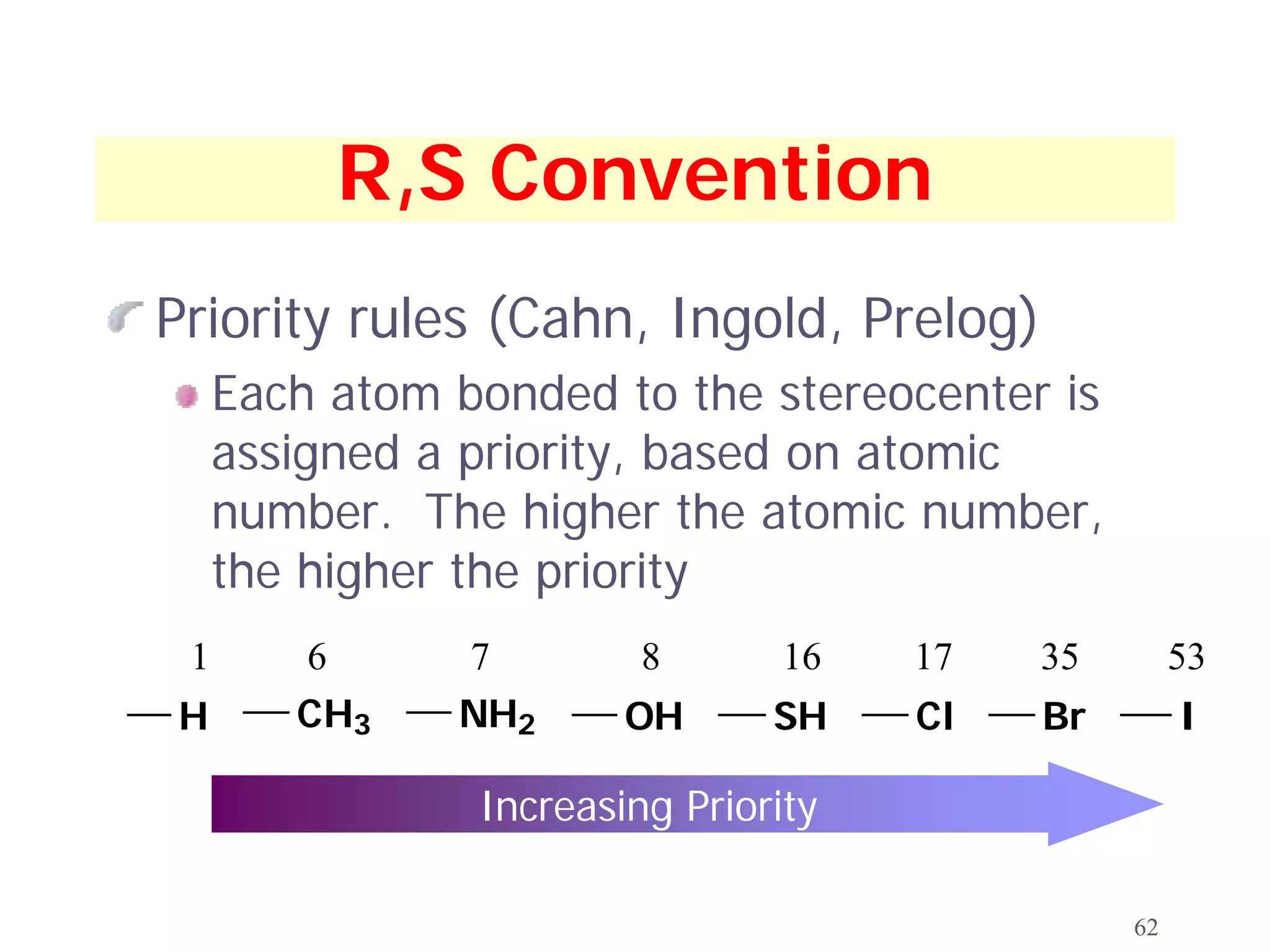
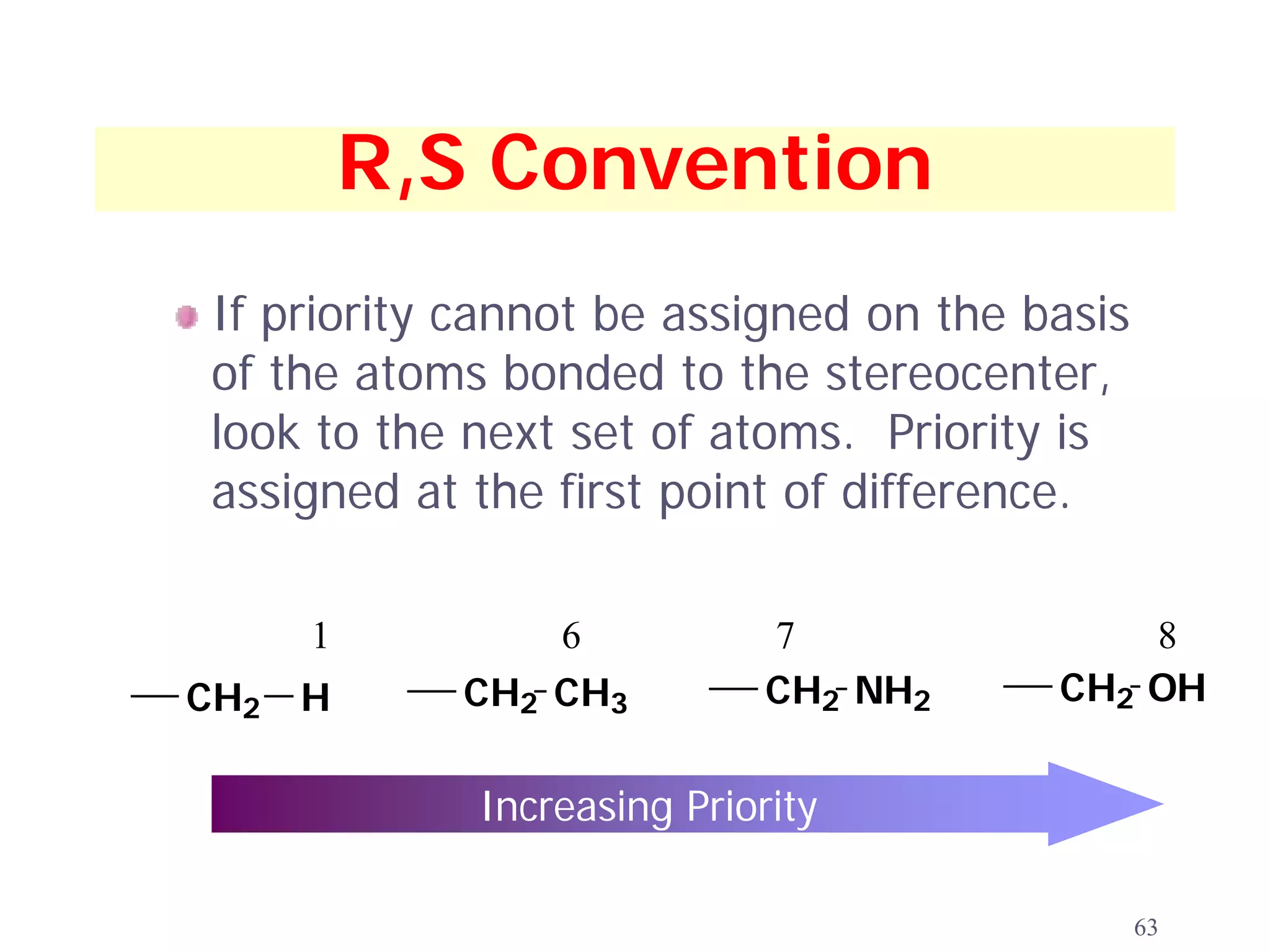
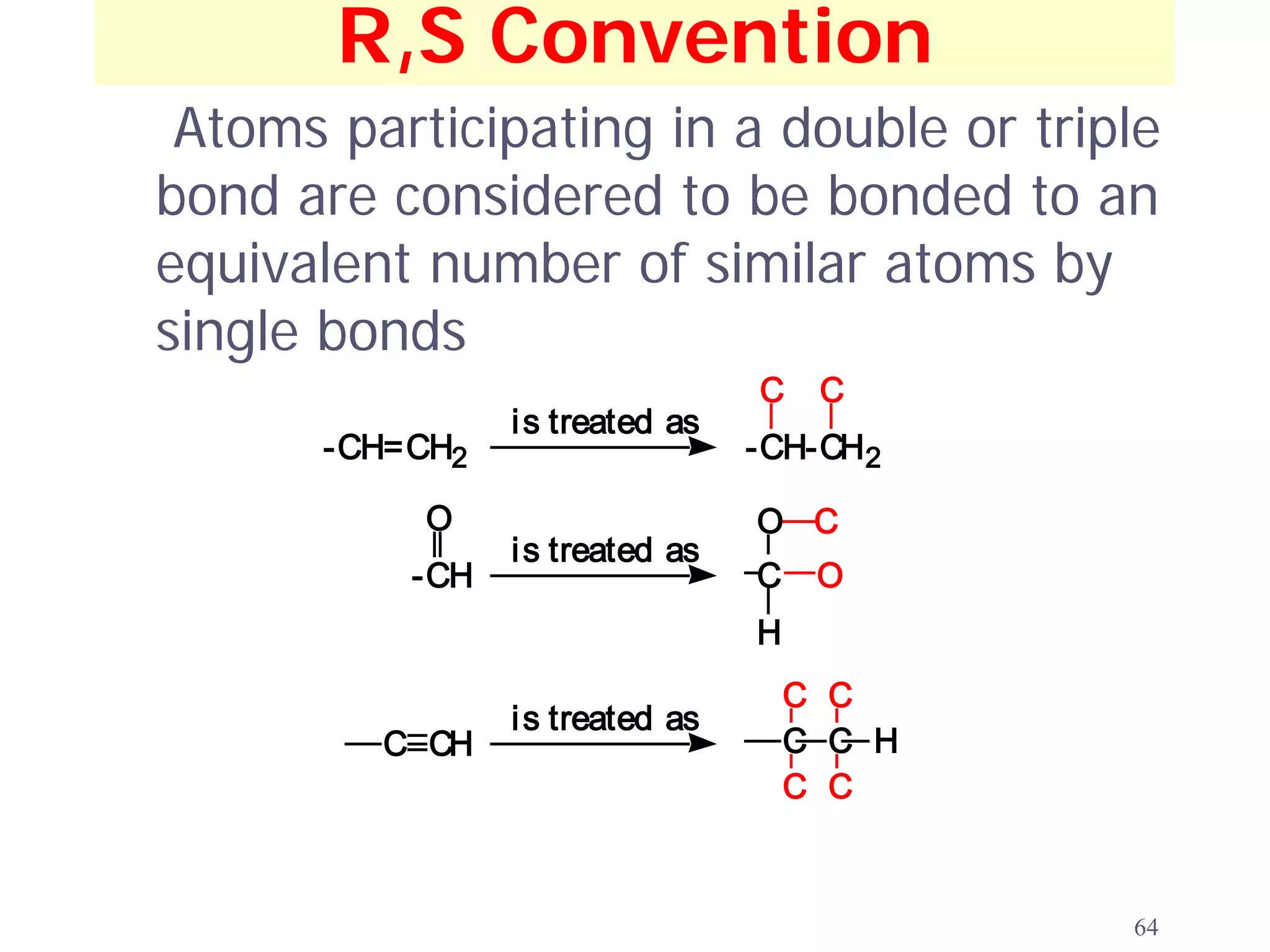
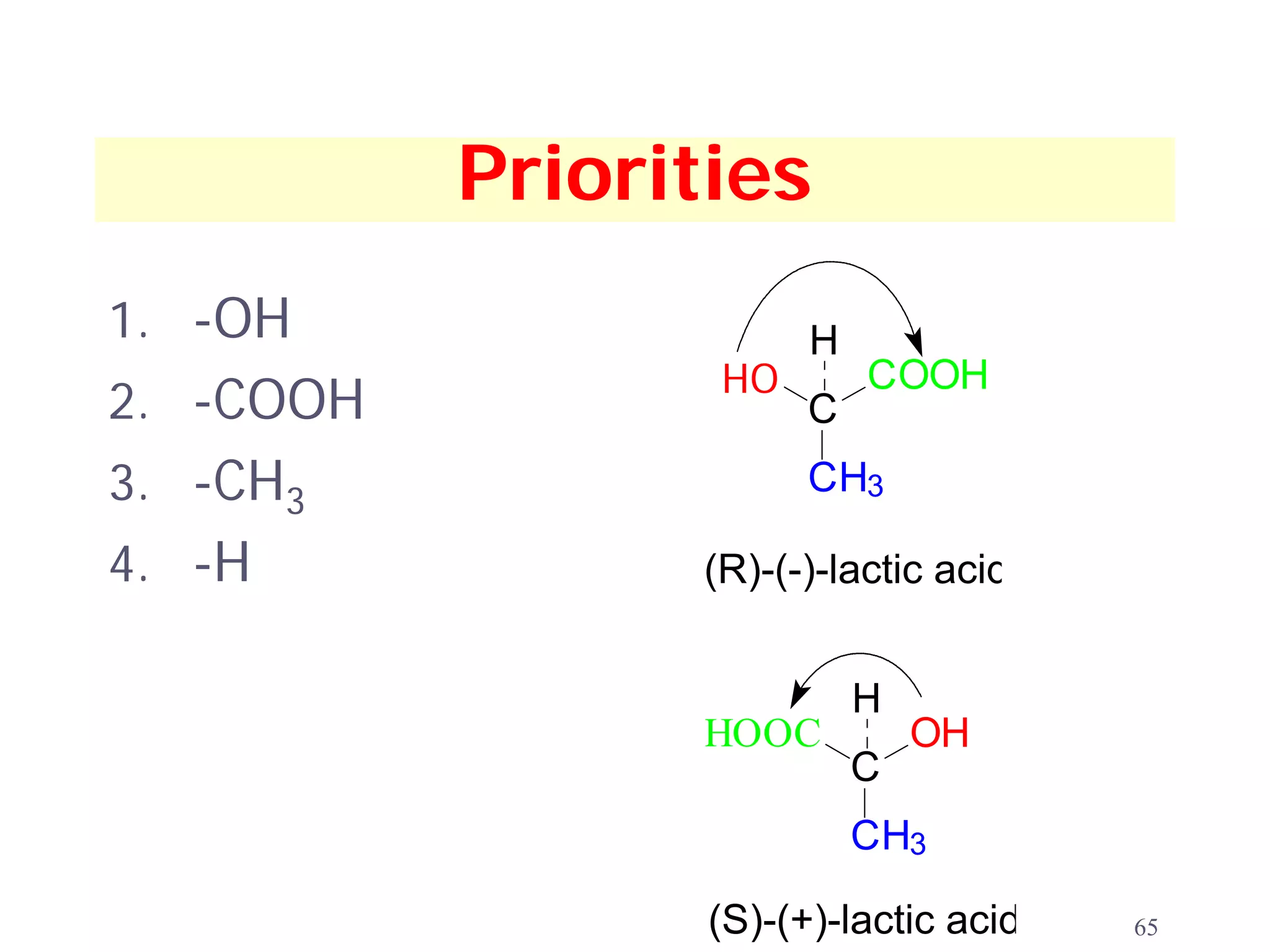
3. The Cahn-Ingold-Prelog priority rules are used to assign R and S configurations based on the atomic number of substituents and their spatial orientation around a stereogenic center.










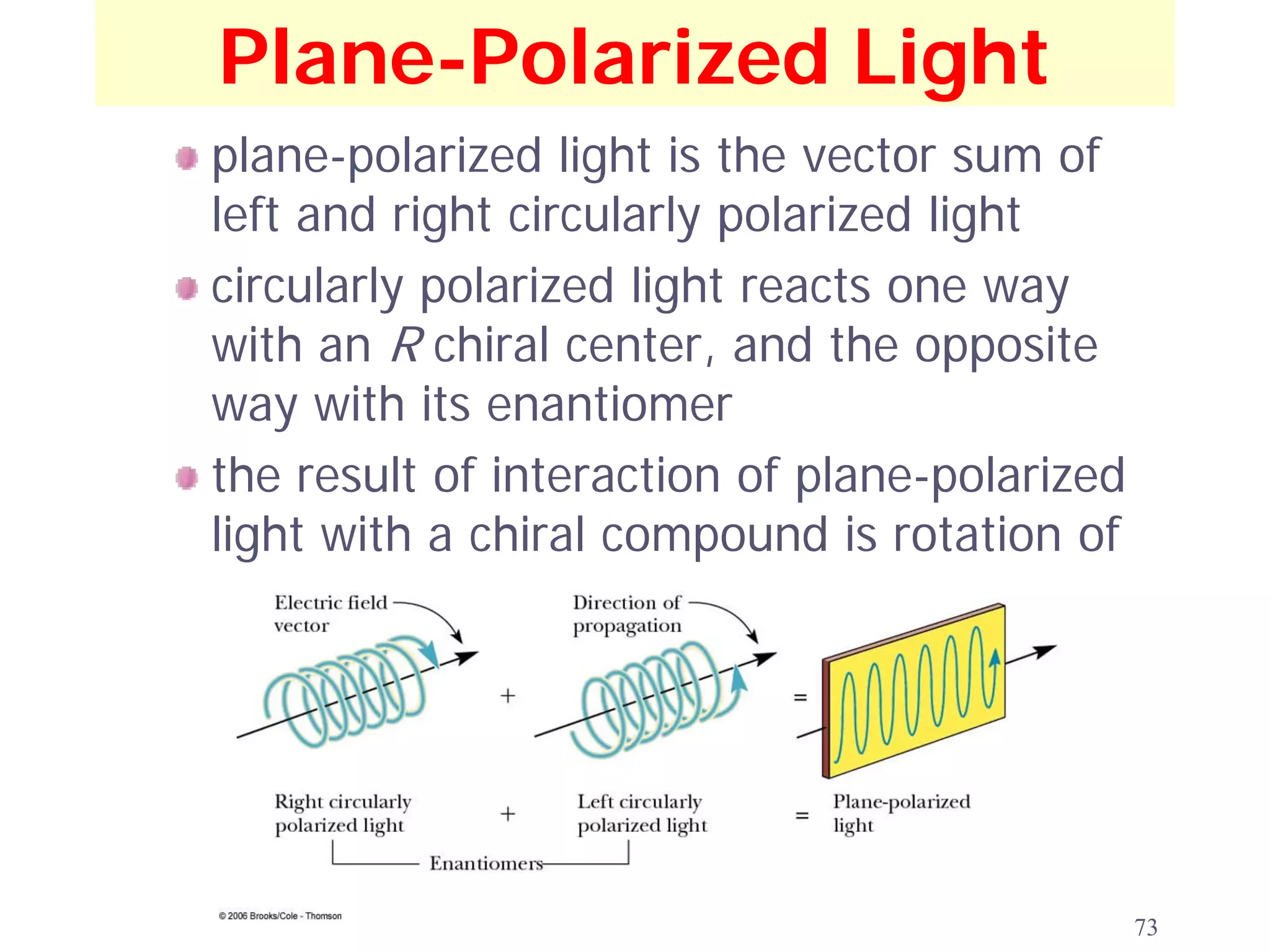
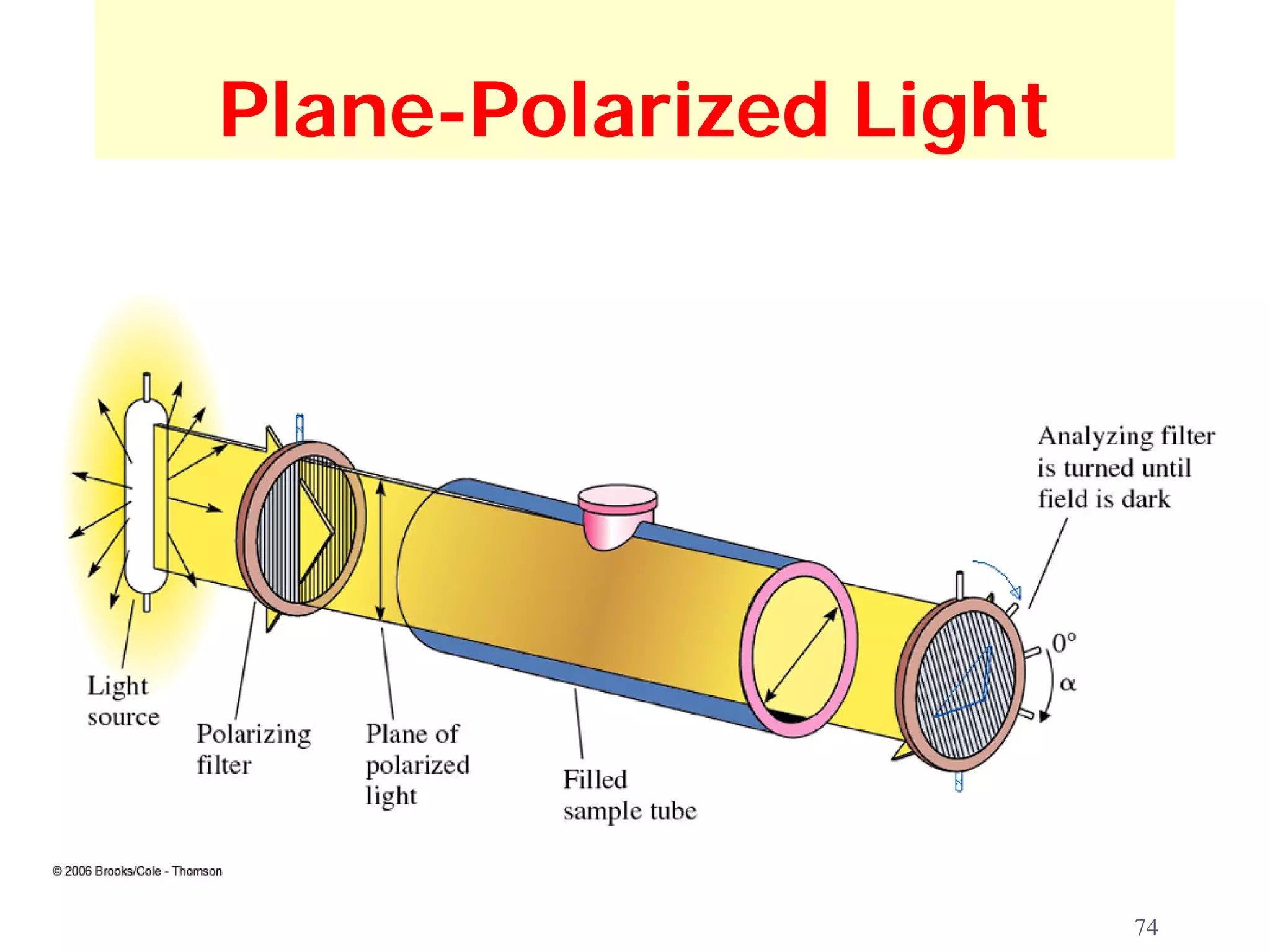
![Optical Activity
observed rotation: the number of degrees, α, through
which a compound rotates the plane of polarized
light
dextrorotatory (+): refers to a compound that
rotates the plane of polarized light to the right
levorotatory (-): refers to a compound that rotates of
the plane of polarized light to the left
specific rotation: observed rotation when a pure
sample is placed in a tube 1.0 dm in length and
concentration in g/mL (density); for a solution,
concentration is expressed in g/ 100 mL
COOH COOH
C H H C
H3 C OH CH3
HO
(S)-(+)-Lacti c aci d (R)-(-)-L actati c aci d
21 21
[ α] D = +2.6° [ α] D = -2.6°
75](https://image.slidesharecdn.com/isomer-100922135826-phpapp01/75/Isomer-Presentation-Examville-com-75-2048.jpg)
![Optical Purity
Optical purity: a way of describing the
composition of a mixture of enantiomers
[α ]sam p l e
Percent opti cal puri ty = x 100
[α ]p u re en an ti o mer
Enantiomeric excess: the difference between
the percentage of two enantiomers in a
mixture
[R] - [S]
Enan ti omeri c excess (ee) = x 100 = %R - %S
[R] + [S]
optical purity is numerically equal to enantiomeric
excess, but is experimentally determined
76](https://image.slidesharecdn.com/isomer-100922135826-phpapp01/75/Isomer-Presentation-Examville-com-76-2048.jpg)

![Specific Rotation
• Specific rotation is a standardized physical constant for
the amount that a chiral compound rotates plane-
polarized light. Specific rotation is denoted by the
symbol [α] and defined using a specific sample tube
length (l, in dm), concentration (c in g/mL), temperature
(25 0C) and wavelength (589 nm).
79](https://image.slidesharecdn.com/isomer-100922135826-phpapp01/75/Isomer-Presentation-Examville-com-79-2048.jpg)
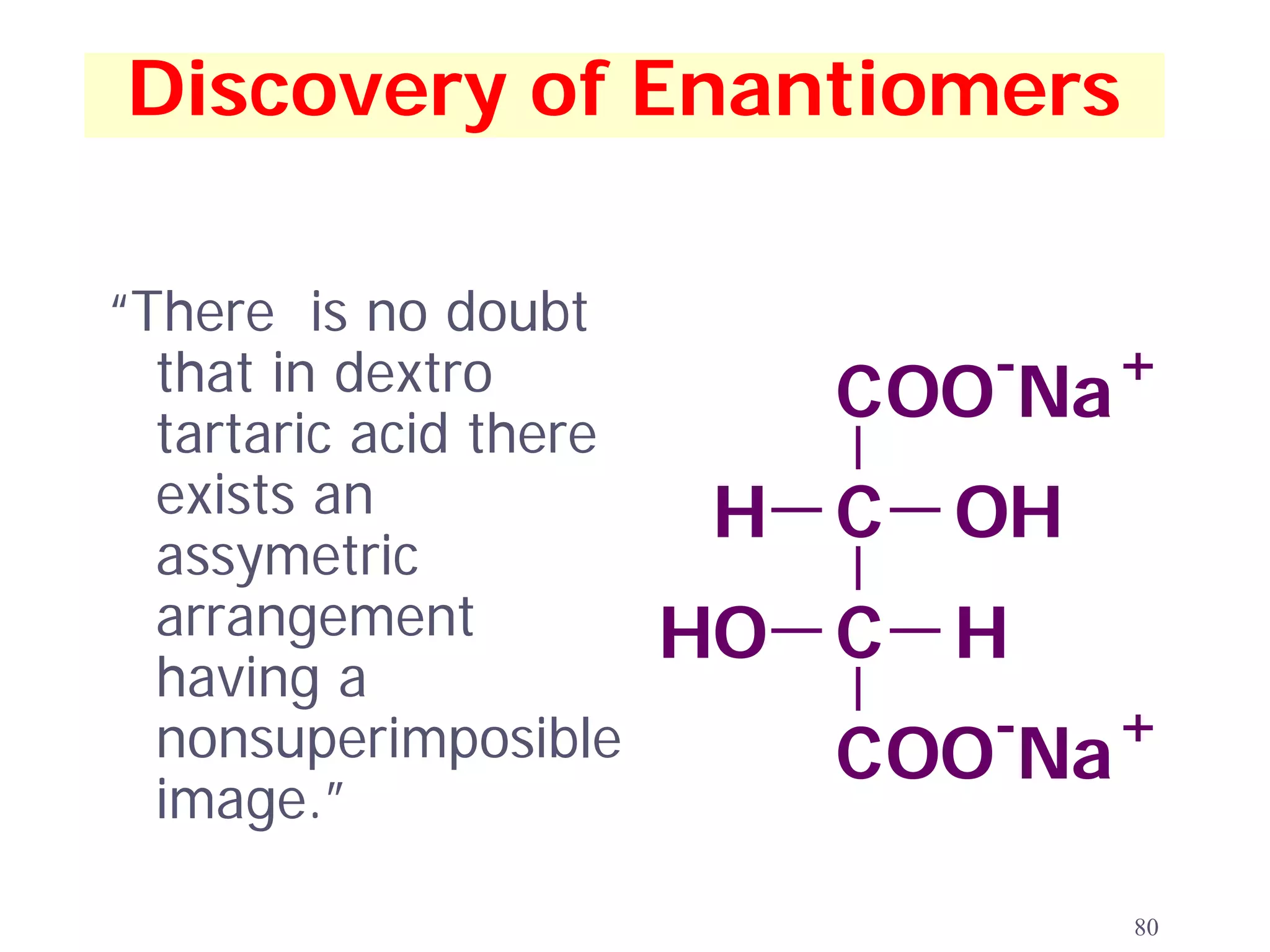
![Tartaric Acid
OH OH OH OH
HOOC H H COOH
H COOH HOOC H
(+)-tartaric acid (-)-tartaric acid
OH OH meso ALSO FOUND
(as a minor component)
HOOC COOH
H H [α]D = 0
more about this
meso -tartaric acid compound later
81](https://image.slidesharecdn.com/isomer-100922135826-phpapp01/75/Isomer-Presentation-Examville-com-81-2048.jpg)

![Tartaric Acid
(-) - tartaric acid (+) - tartaric acid
[α]D = -12.0o [α]D = +12.0o
mp 168 - 170o mp 168 - 170o
solubility of 1 g solubility of 1 g
0.75 mL H2O 0.75 mL H2O
1.7 mL methanol 1.7 mL methanol
250 mL ether 250 mL ether
insoluble CHCl3 insoluble CHCl3
d = 1.758 g/mL d = 1.758 g/mL
meso - tartaric acid
[α ]D = 0 o solubility of 1 g
mp 140o 0.94 mL H2O
d = 1.666 g/mL insoluble CHCl3
89](https://image.slidesharecdn.com/isomer-100922135826-phpapp01/75/Isomer-Presentation-Examville-com-89-2048.jpg)