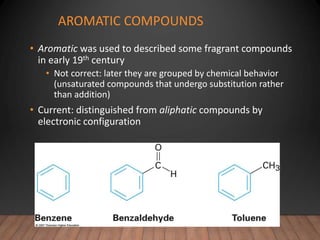
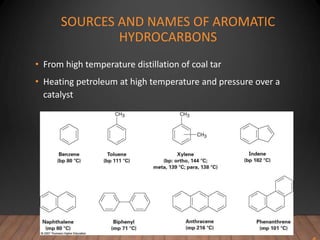
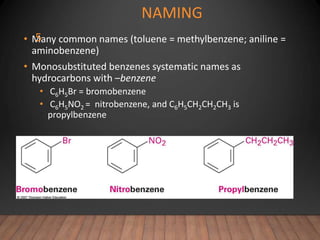
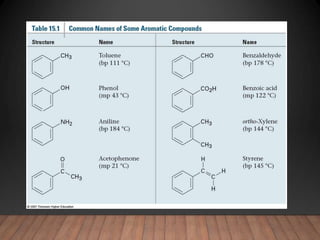
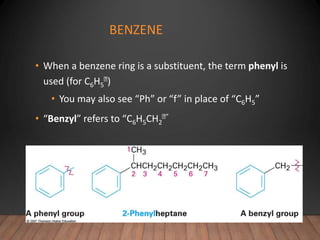
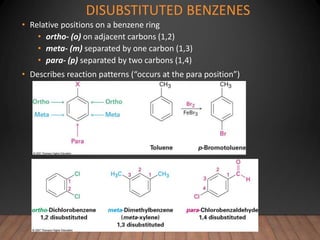
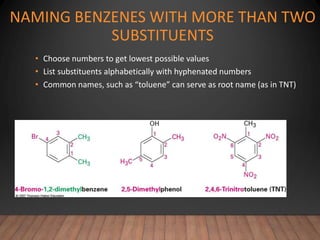
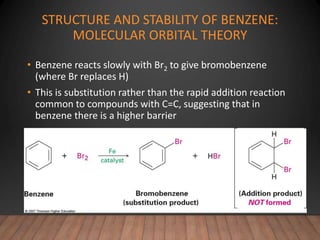
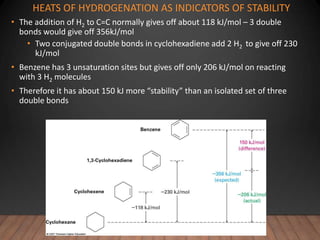
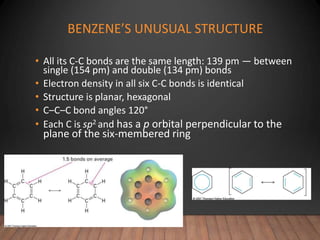
- Aromatic compounds are characterized by a cyclic, conjugated ring system with delocalized pi electrons. This allows them to undergo substitution rather than addition reactions.
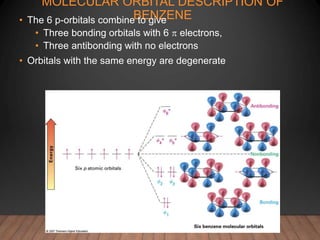
- Benzene is the prototypical aromatic compound. Its 6 pi electrons are delocalized across the ring, giving it extra stability compared to isolated double bonds. This is explained by molecular orbital theory.
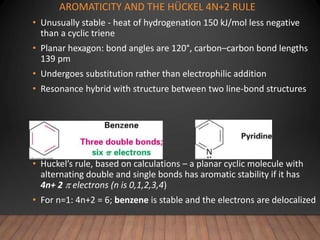
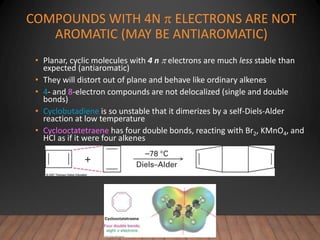
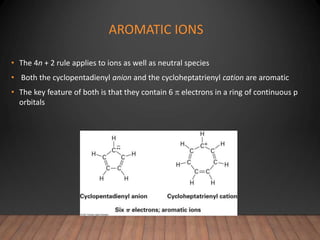
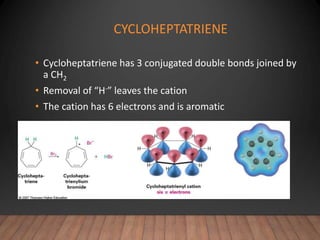
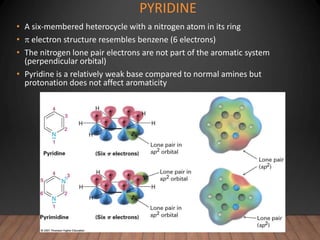
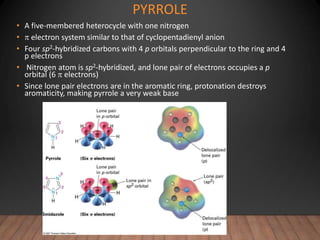
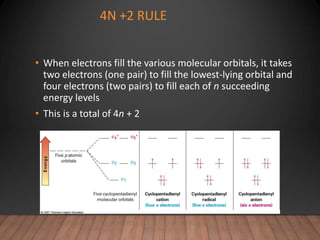
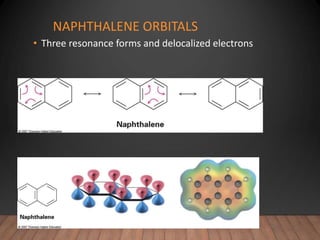
- The Hückel rule states that monocyclic compounds with 4n+2 pi electrons are aromatic. Heterocycles like pyridine and pyrrole can also be aromatic. Polycyclic aromatic compounds have multiple fused rings.