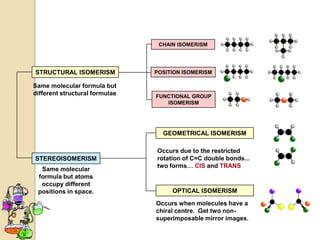
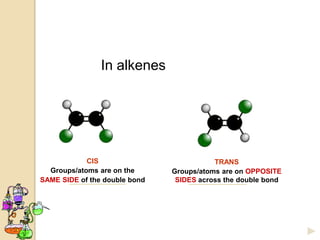
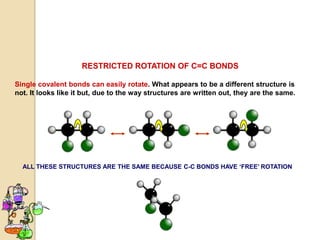
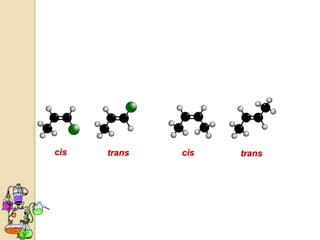
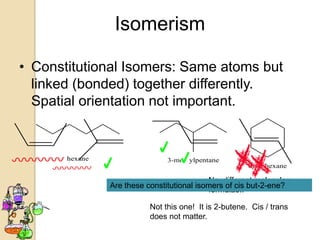
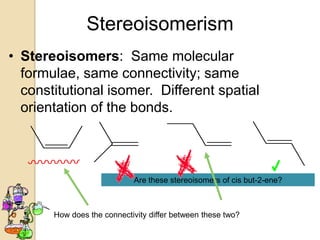
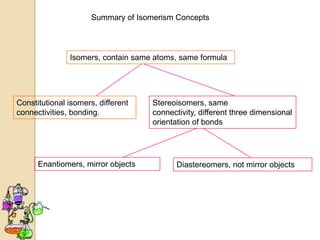
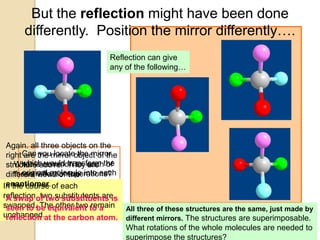
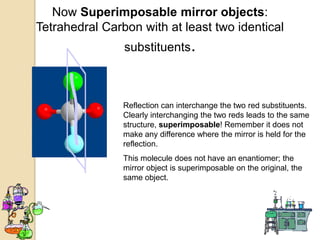
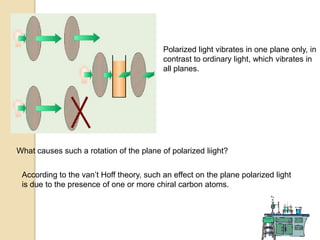
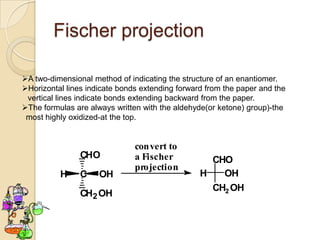
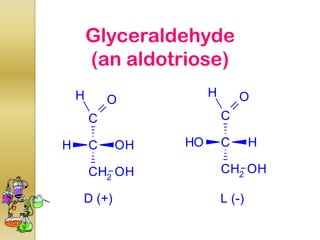
The document discusses different types of isomerism including structural isomerism, stereoisomerism, and constitutional isomerism. It explains that stereoisomers have the same molecular formula and connectivity but different spatial orientations of bonds. Specifically, it defines enantiomers as stereoisomers that are non-superimposable mirror images, and diastereomers as stereoisomers that are not mirror images. The document also discusses restricted rotation of double bonds leading to cis-trans isomers and the use of Fischer projections to represent stereoisomers.