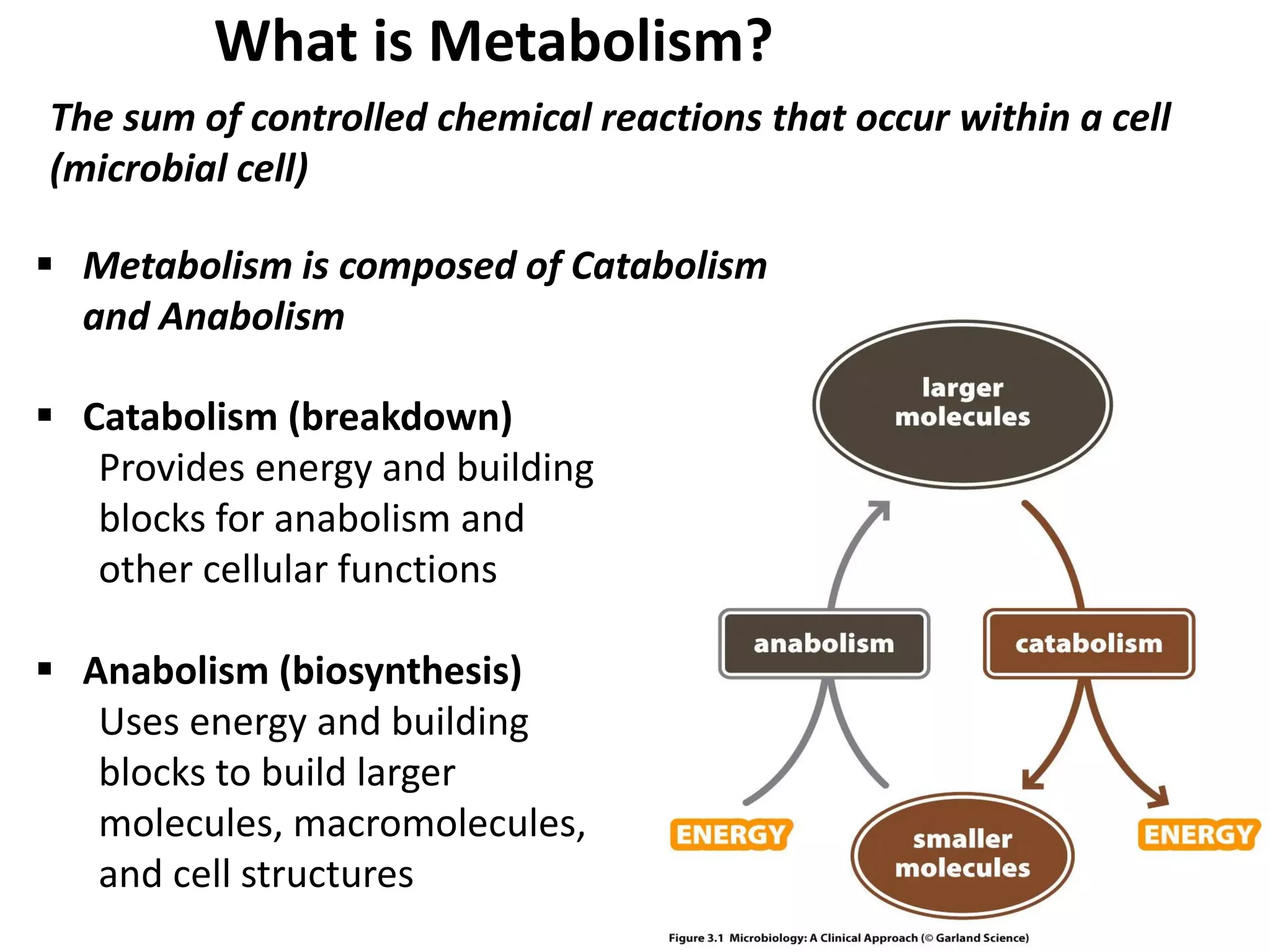
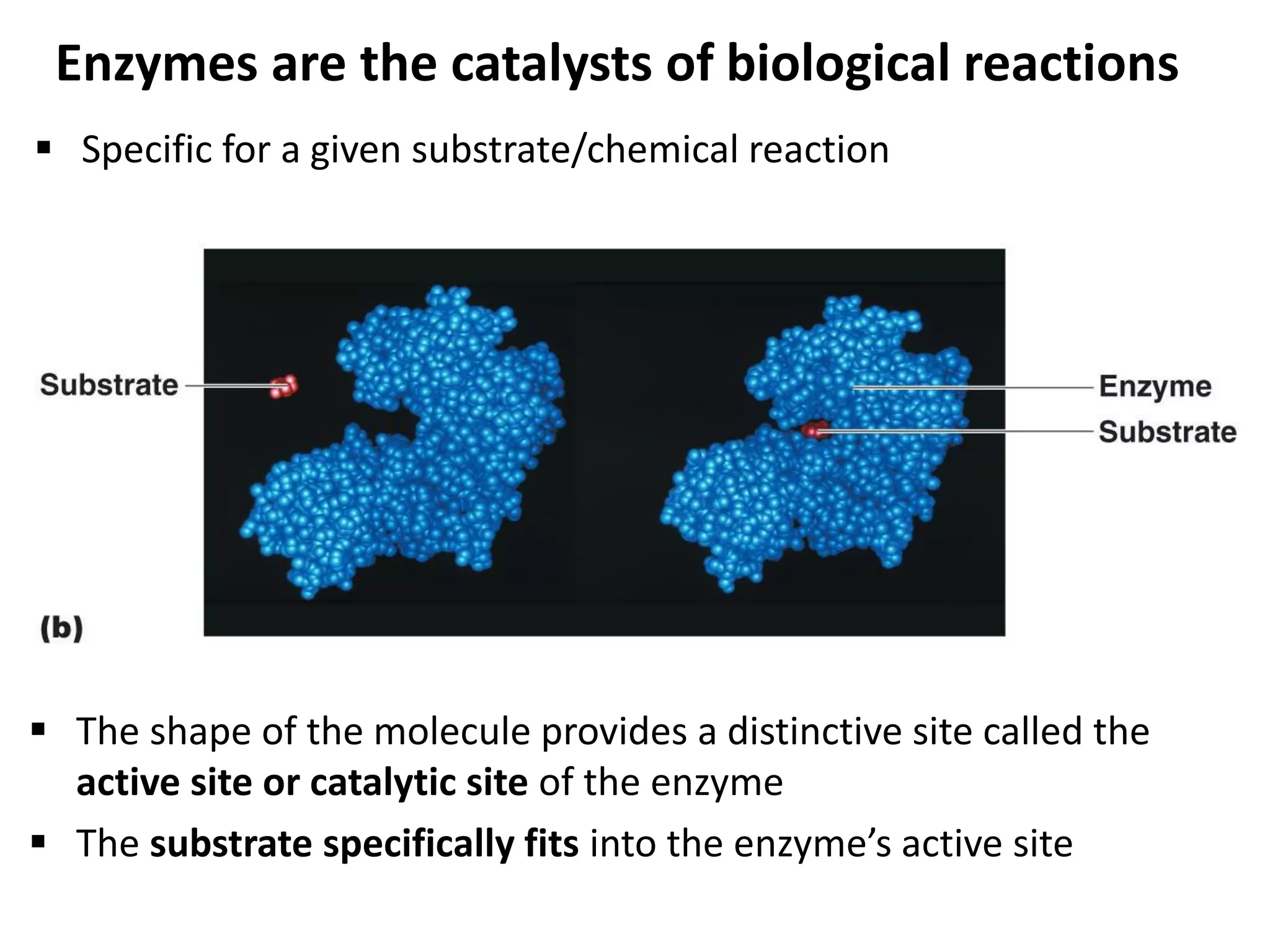
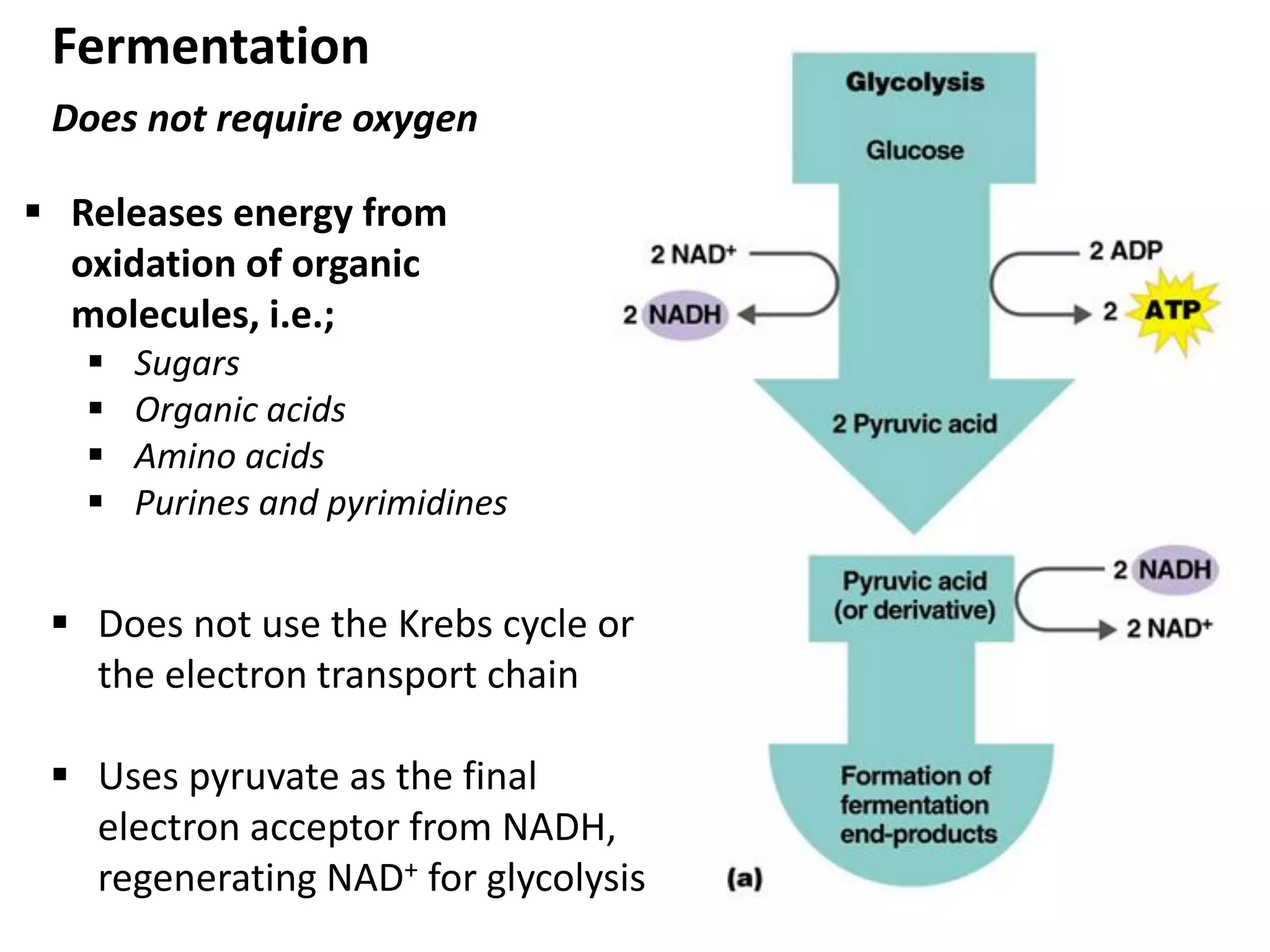
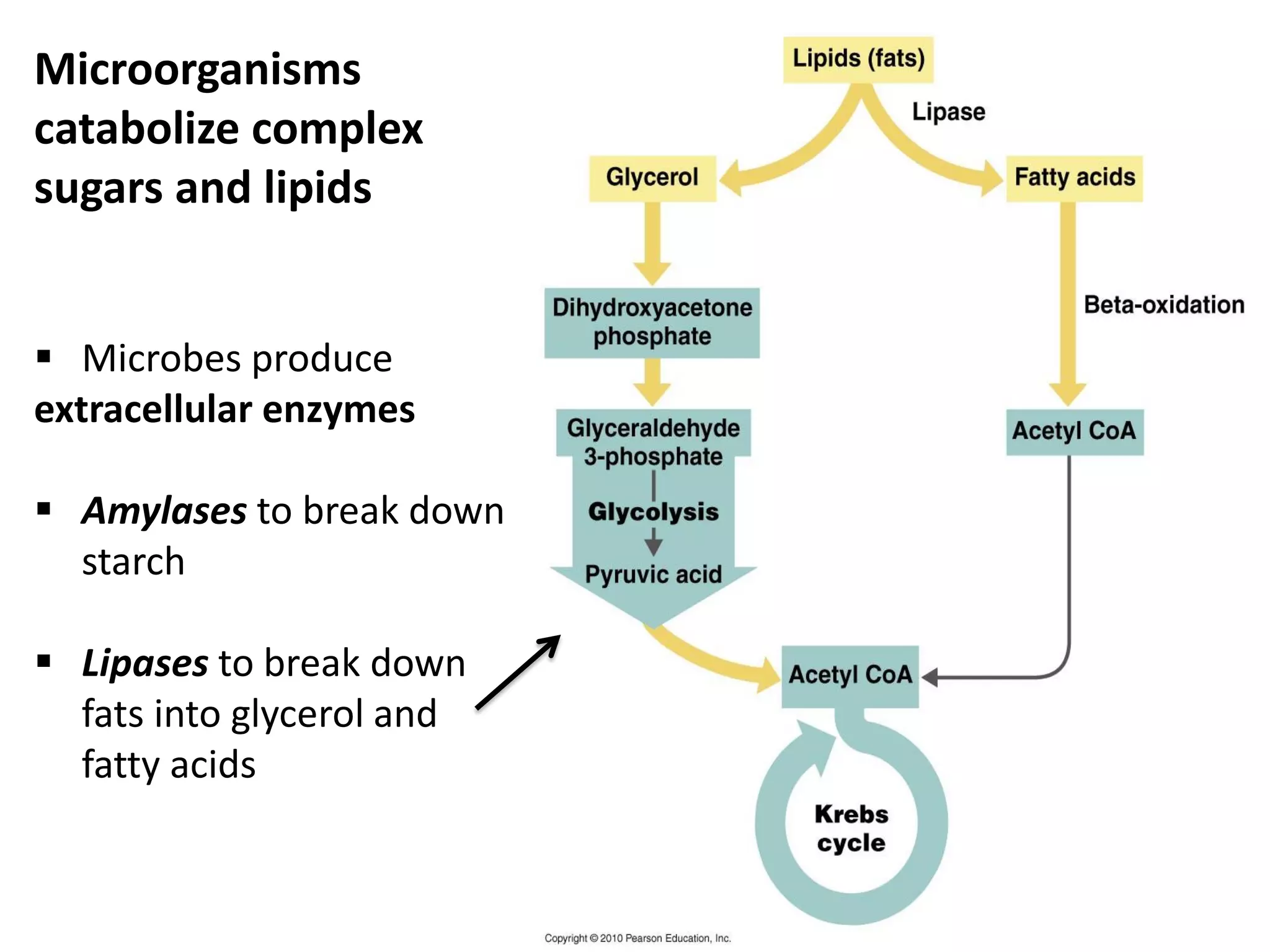
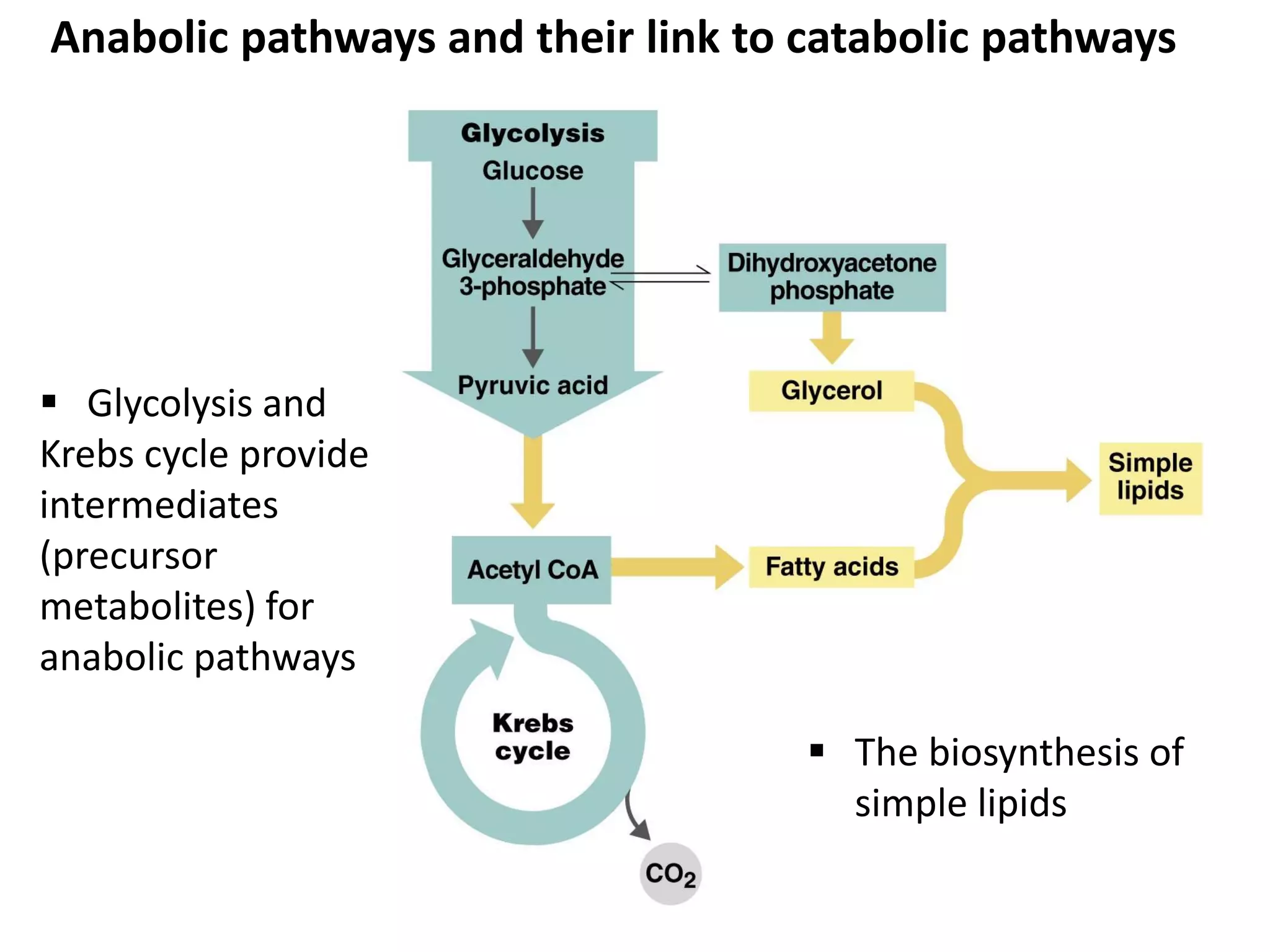
Metabolism consists of catabolic and anabolic reactions that are organized into metabolic pathways. Catabolic pathways break down molecules to generate energy in the form of ATP via redox reactions. Anabolic pathways use this energy to synthesize larger molecules. Enzymes catalyze reactions and cells regulate pathways through feedback inhibition. Microbes metabolize carbohydrates, proteins, lipids, and nucleic acids to generate energy and building blocks through both aerobic and anaerobic pathways. Metabolic tests can be used to distinguish between microbial species in the laboratory.