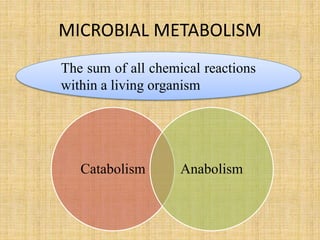
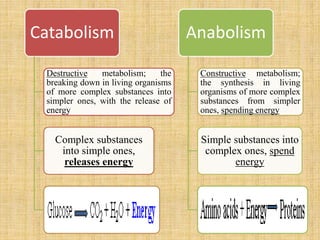
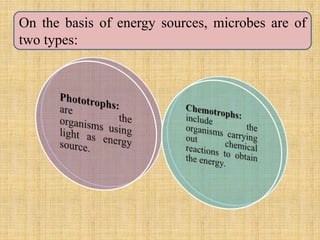
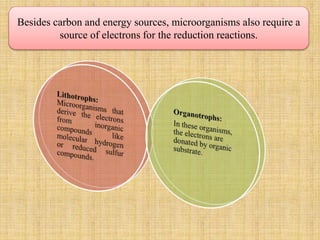
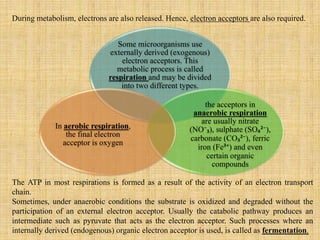
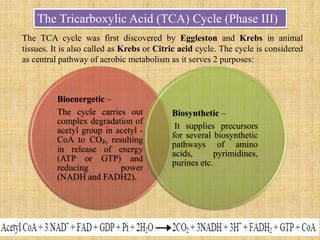
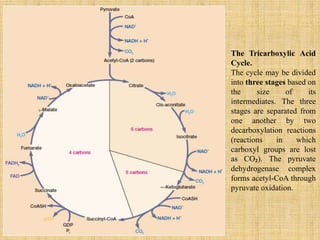
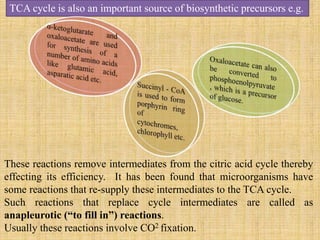
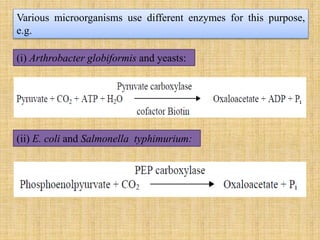
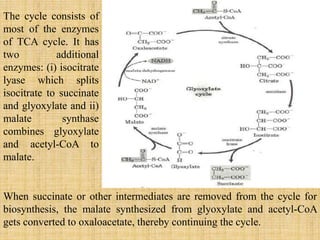
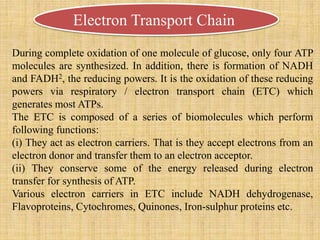
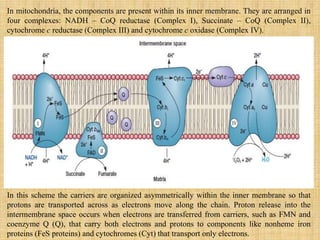
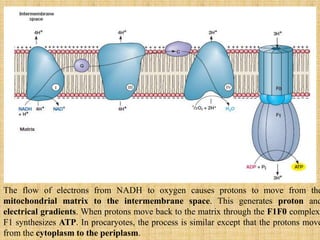
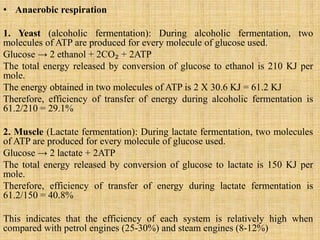
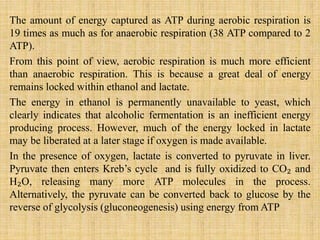
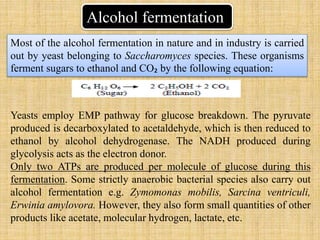
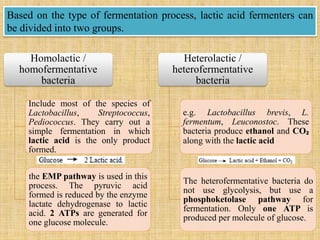
1. Microbial metabolism involves catabolic and anabolic reactions. Catabolism breaks down complex substances into simple ones with energy release, while anabolism uses this energy to synthesize complex substances from simple ones.
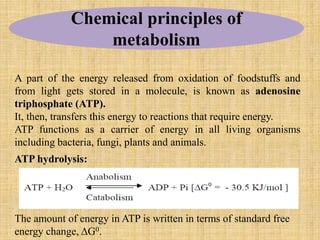
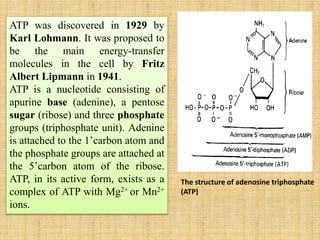
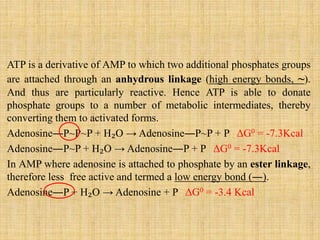
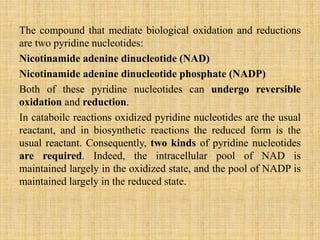
2. ATP acts as the universal energy carrier in living organisms. It stores and transfers energy released during catabolism to drive anabolic reactions.
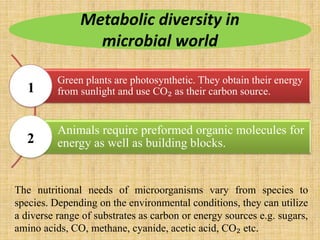
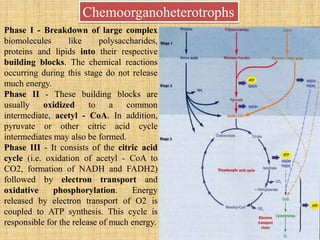
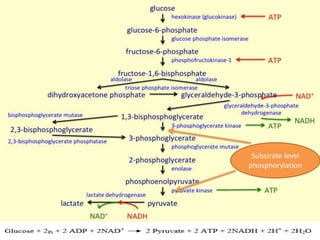
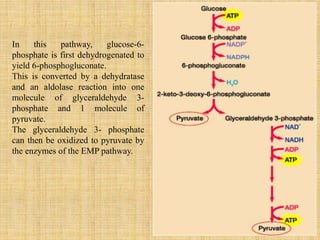
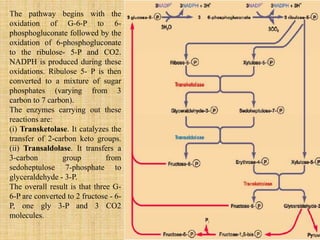
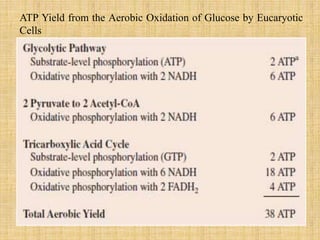
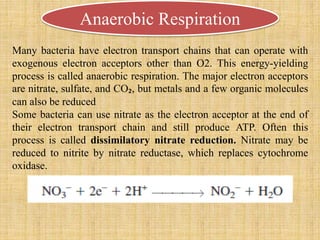
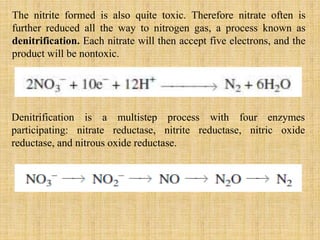
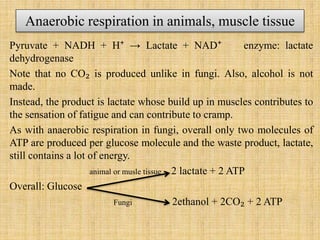
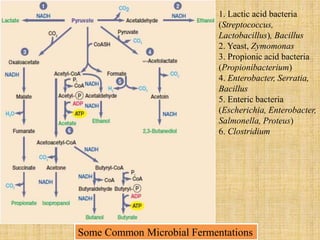
3. Microbes obtain energy and carbon through various metabolic pathways like glycolysis, TCA cycle, and oxidative phosphorylation during aerobic respiration or fermentation during anaerobic respiration.