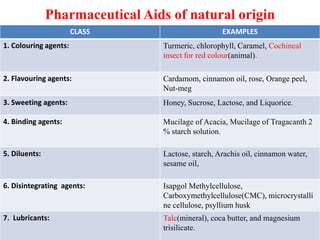
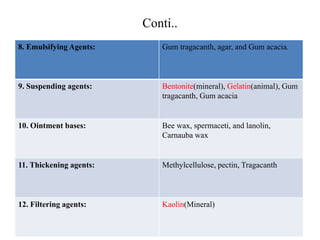
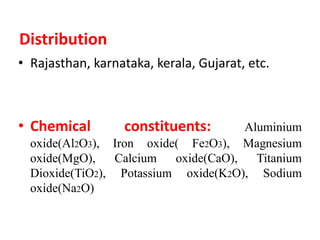
Pharmaceutical aids are substances added to pharmaceutical preparations that have little or no therapeutic effect but aid in the manufacturing and preservation of drugs. They include diluents, binders, coatings, and preservatives that are often of plant, animal or mineral origin like talc, kaolin, and bentonite. Talc, kaolin, and bentonite are commonly used pharmaceutical aids with specific properties that allow their use as lubricants, absorbents, or suspending agents in drug formulations.