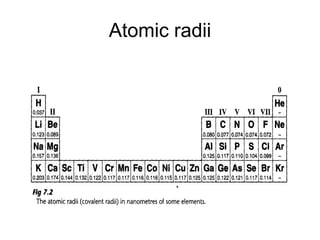
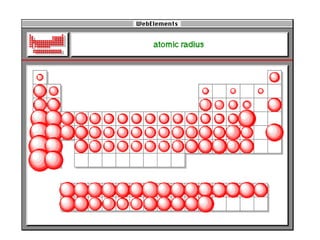
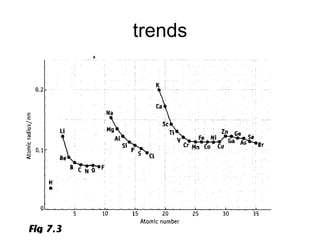
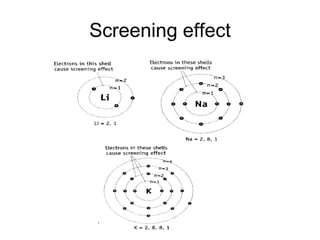
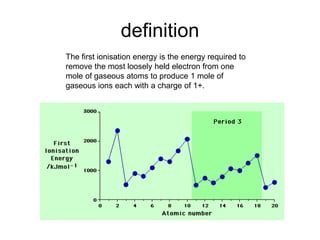
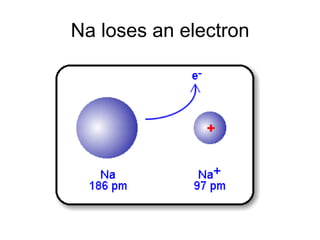
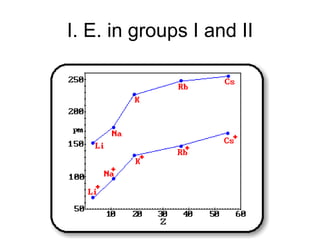
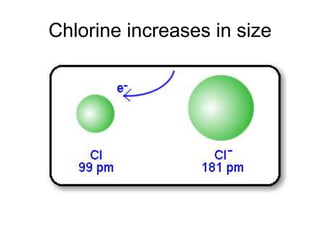
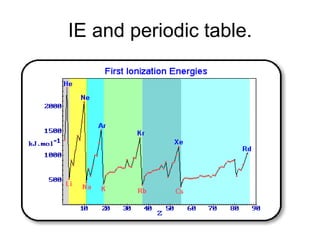
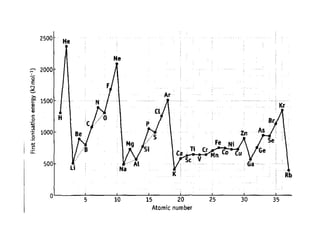
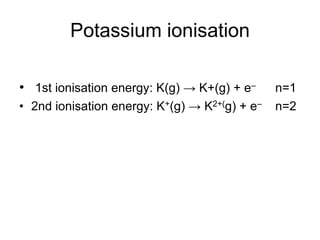
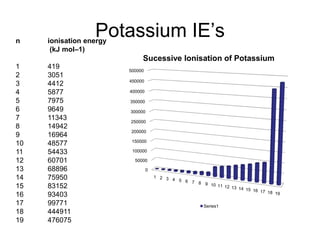
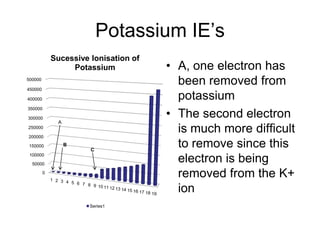
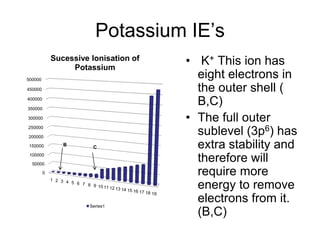
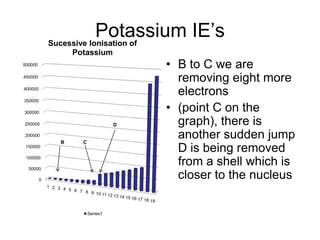
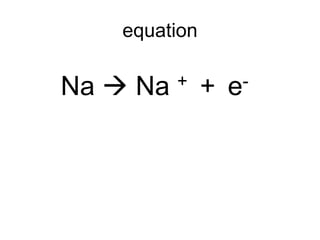The document discusses atomic radii and ionization energy trends across periods and down groups of the periodic table. It explains that atomic radii generally decrease across periods as nuclear charge increases, while increasing down groups as additional electrons occupy farther shells. Ionization energy also typically increases across periods and decreases down groups. Exceptions are noted for beryllium and nitrogen. Evidence for electronic energy levels is shown through successive ionization energies of potassium that indicate full and half-filled shells require more energy to further ionize.