The document provides an overview of oxidation and reduction concepts including:
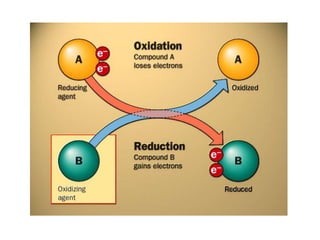
- Oxidation involves loss of electrons while reduction involves gain of electrons.
- Examples of oxidation and reduction reactions are given for sodium-chlorine, magnesium-oxygen, and zinc-copper sulfate.
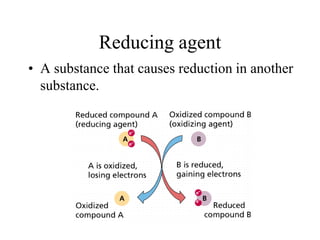
- Oxidizing and reducing agents are defined as substances that cause oxidation or reduction in other substances.
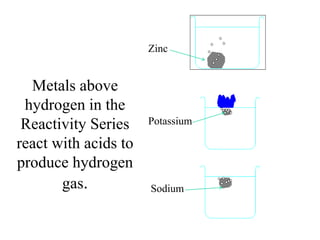
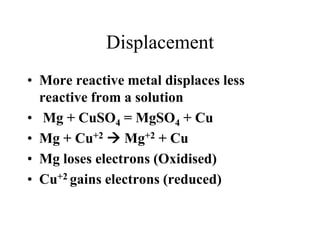
- The electrochemical series orders metals by their tendency to be oxidized.
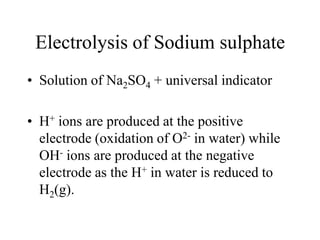
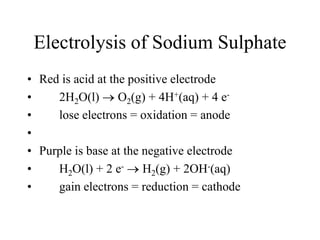
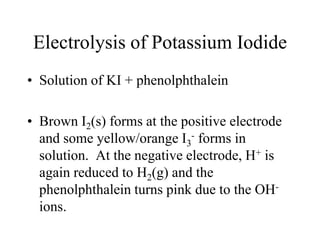
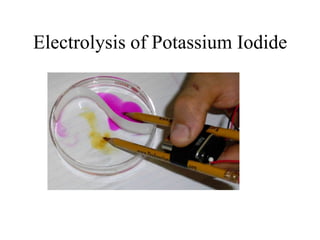
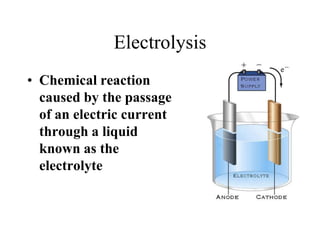
- Electrolysis and examples like copper plating and extracting copper from scrap iron using electrolysis are summarized.



![examples
• Sodium + chlorine sodium chloride
• Na + Cl Na+ + Cl• Na loses an electron [oxidised]
• Cl gains an electron [reduced]](https://image.slidesharecdn.com/1-140308201119-phpapp01/85/1-5-oxidation-and-reduction-4-320.jpg)
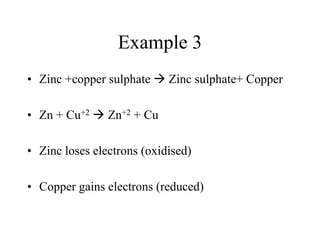
![Example 2
• Magnesium +oxygen magnesium oxide
• Mg + O MgO
• Mg Mg+2 loses 2 electrons [oxidation]
• O O-2 gains 2 electrons [reduction]](https://image.slidesharecdn.com/1-140308201119-phpapp01/85/1-5-oxidation-and-reduction-7-320.jpg)

![•
•
•
•
•
•
•
Oxidation is loss of electrons;
Reduction is gain of electrons
CuO + H2 Cu + H2O
CuO Cu+2 and O-2
Cu+2 Cu [gains 2 electrons] reduced
H2 H2+2[loses 2 electrons] oxidised
O-2 O-2 [ no change]](https://image.slidesharecdn.com/1-140308201119-phpapp01/85/1-5-oxidation-and-reduction-11-320.jpg)



![Oxidation number rules 3
•
•
•
•
•
•
Charges of all elements in a compound = 0
CuSO4
Cu = +2
S = +6
O4 = -8 [O = -2]
Total = +2 +6 –8 = 0](https://image.slidesharecdn.com/1-140308201119-phpapp01/85/1-5-oxidation-and-reduction-15-320.jpg)
![Oxidation number rules 4
• Oxygen = -2
• Exceptions are
• peroxides O = -1 [H2O2, Na2O2 ]
• OF2 O = +2, F = -1](https://image.slidesharecdn.com/1-140308201119-phpapp01/85/1-5-oxidation-and-reduction-16-320.jpg)

![Oxidation number rules 6
• Halogens [ Cl, F, I, Br] are always –1
except when joined to more electropositice
element
• Cl2O
• Cl = +1, O = -2](https://image.slidesharecdn.com/1-140308201119-phpapp01/85/1-5-oxidation-and-reduction-18-320.jpg)
![Oxidation number rules 7
• In a complex ion the sum of all the charges
= the chartge on the ion.
• SO4-2
• S = +6, O4 = -8 [O = -2]
• +6 –8 = -2](https://image.slidesharecdn.com/1-140308201119-phpapp01/85/1-5-oxidation-and-reduction-19-320.jpg)


![Metals
•
•
•
•
•
•
•
•
•
•
•
•
King [K]
Neptune [Na]
Caught [Ca]
Many [Mg]
Angry [Al]
Zulus [Zn]
Fighting [Fe]
Police [Pb]
Constables [Cu]
Having [Hg]
Asthma [Ag]
Attacks [Au]](https://image.slidesharecdn.com/1-140308201119-phpapp01/85/1-5-oxidation-and-reduction-22-320.jpg)




![Swimming pools
• 2. The addition of sodium chlorate(I) [sodium
hypochlorite]:
• NaOCl (s) + H2O (l) Na+ (aq) + OH− (aq) + HOCl (aq)
• Nowadays chlorine is not used, mainly on grounds of
safety. Pools are sterilized with chlorine compounds,
which produce chloric(I) acid when they dissolve in
water. These compounds act in essentially the same
way as chlorine. Sodium chlorate(I) is one such
compound.](https://image.slidesharecdn.com/1-140308201119-phpapp01/85/1-5-oxidation-and-reduction-29-320.jpg)

![Definitions
•
Electrolyte - liquid in which electrolysis takes place. Usually an ionic solution but it can also be a
fused [melted] ionic compound
•
•
•
•
•
Anode - positive electrode. Positive because the battery sucks electrons out of it
•
Cation - positive ion. Called cation because it is attracted to the opposite charge of the cathode.
•
Inert Electrodes - do not react with the electrolyte Graphite and Pt
•
Active electrodes - react with electrolyte e.g. Copper and iron
Cathode. Negative electrode. Negative because the battery pumps electrons into it.
Anion - negative ion. Called anion because it is attracted to the opposite charge of the anode](https://image.slidesharecdn.com/1-140308201119-phpapp01/85/1-5-oxidation-and-reduction-32-320.jpg)
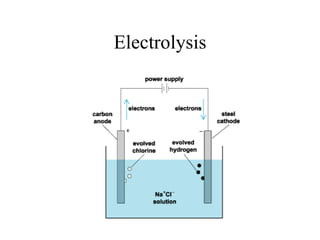

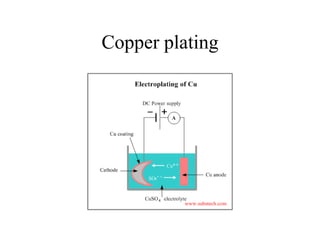
![Copper Plating
Anode reaction
• Cu(s) = Cu2+(aq) + 2e• Anode loses mass as copper dissolves off
• Impurities [Au, Ag, Pt etc.] fall to bottom
Cathode reaction
• Cu2+(aq) + 2e- = Cu(s)
• Cathode gains mass as Cu is deposited on it
• Cu is 99.9% pure](https://image.slidesharecdn.com/1-140308201119-phpapp01/85/1-5-oxidation-and-reduction-36-320.jpg)
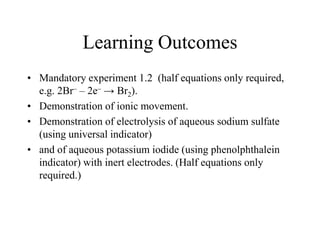
![Ionic Movement
• During electrolysis of a solution of Copper Chromate in
dil. Hydrochloric acid, positive ions (cations) are attracted
to the negative electrode (cathode) and negative ions
(anions) are attracted to the positive electrode (anode). If
these ions are coloured, their movement may be observed
visually.
• Examples of coloured ions include;
• copper(II) [Cu2+] - blue
• chromate(VI) [CrO42- ] – yellow](https://image.slidesharecdn.com/1-140308201119-phpapp01/85/1-5-oxidation-and-reduction-38-320.jpg)