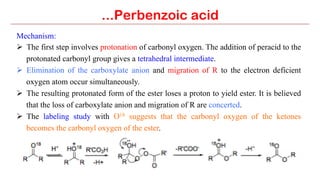
Perbenzoic acid and related peracids such as m-chloroperbenzoic acid are useful oxidizing agents that can perform epoxidation of alkenes, oxidation of amines and thioethers, and Baeyer-Villiger oxidation of ketones to esters. Perbenzoic acid is prepared in situ by oxidation of benzoic acid with hydrogen peroxide and can carry out stereospecific epoxidation of alkenes through a concerted mechanism involving oxygen addition and proton transfer. Common applications of perbenzoic acid include epoxidation, oxidation of functional groups, and Baeyer-