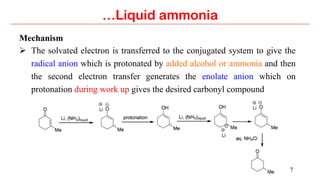
The document discusses the use of liquid ammonia for organic reductions, where dissolved alkali metals generate solvated electrons that can reduce conjugated double/triple bonds, unsaturated ketones, and aromatic compounds through a mechanism involving radical anions and carbanions. Specific applications covered include the reduction of conjugated dienes, α,β-unsaturated ketones to saturated carbonyls/alcohols, and aromatic compounds (Birch reduction) to 1,4-cyclohexadienes. The role of alcohols in these reductions is also explained.