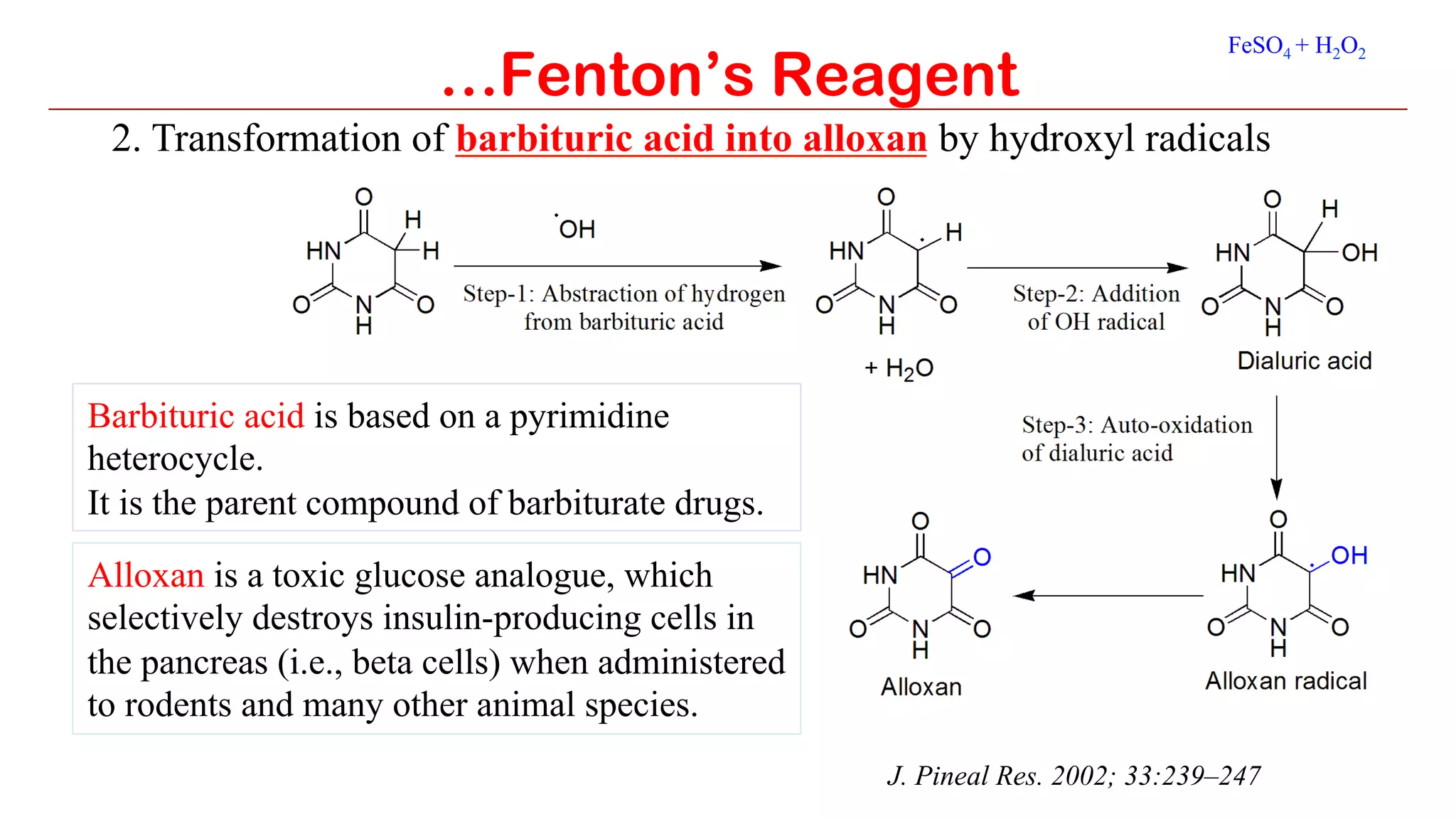
Fenton's reagent is a solution of hydrogen peroxide and ferrous ion that generates hydroxyl radicals and is used to oxidize organic compounds. It consists of iron(II) sulfate and hydrogen peroxide and causes the disproportionation of hydrogen peroxide through a redox cycle to produce hydroxyl and hydroperoxyl radicals. These radicals then engage in secondary reactions to rapidly oxidize contaminants into carbon dioxide and water. Some applications of Fenton's reagent include the hydroxylation of arenes like benzene to phenol, converting barbituric acid to the toxic compound alloxan, and oxidizing glycerol to a mixture of glyceraldehyde and dihydroxyacetone known as glycerose.