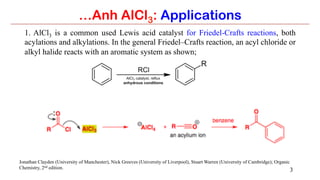
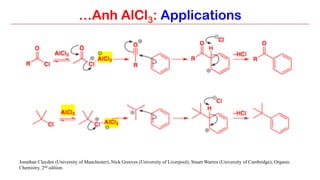
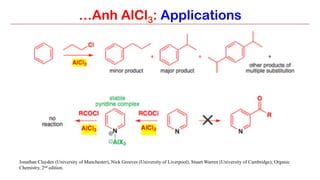
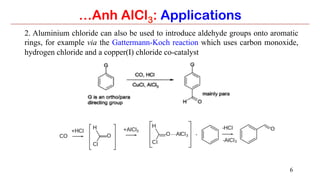
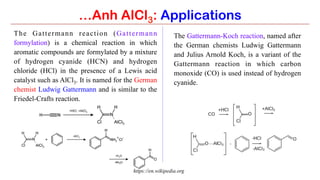
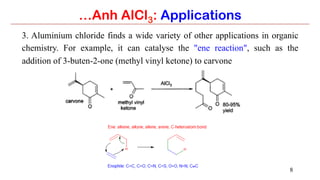
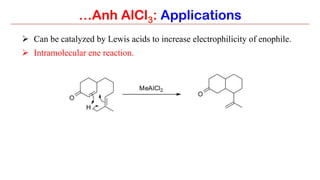
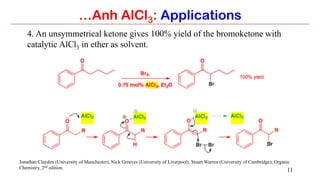
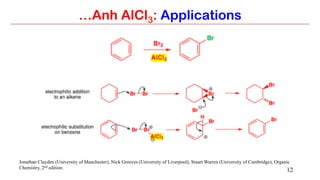
Anhydrous aluminium chloride (AlCl3) is a white solid that is an important Lewis acid catalyst. It is commonly used to catalyze Friedel-Crafts reactions and can introduce aldehyde groups or catalyze ene reactions. AlCl3 also catalyzes the addition of bromine to ketones in ether solvent to give 100% yields of bromoketones.