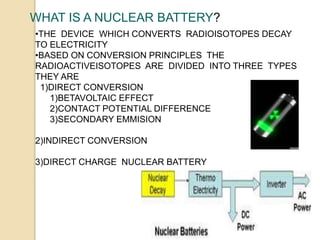
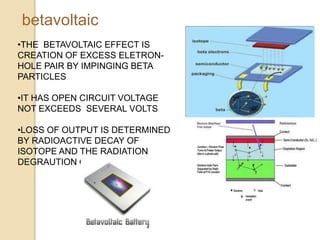
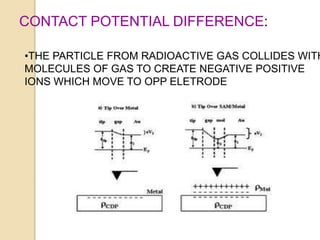
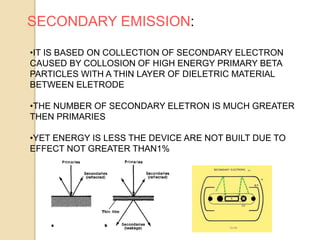
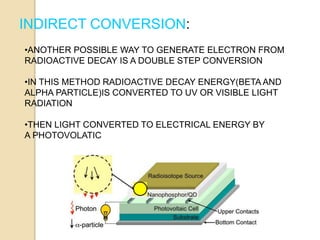
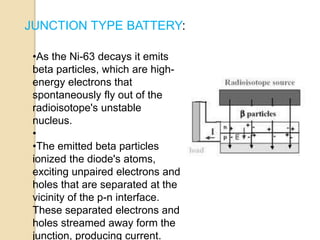
Nuclear batteries convert the decay of radioisotopes into electricity, providing a reliable and long-term power source with a lifespan of at least ten years. They work through various mechanisms including betavoltaic effects and direct charge collection, though their use of radioactive materials raises safety and economic concerns. Despite high initial production costs and size limitations, the potential applications across fields like medicine, space exploration, and military technology highlight their innovative advantages over conventional chemical batteries.