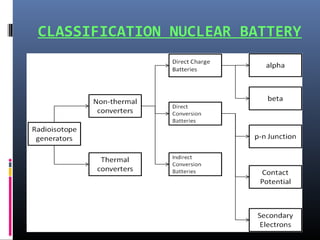
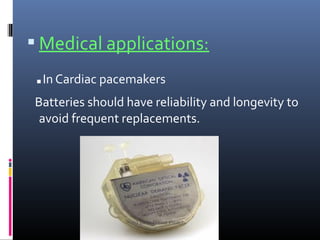
Nuclear batteries harness energy from radioactive isotopes, offering a compact and efficient alternative to traditional power sources. They generate electricity through beta particles, providing long lifespan and high energy density while producing minimal waste. Applications range from space missions to medical devices, though high initial costs and regulatory challenges remain obstacles for widespread adoption.