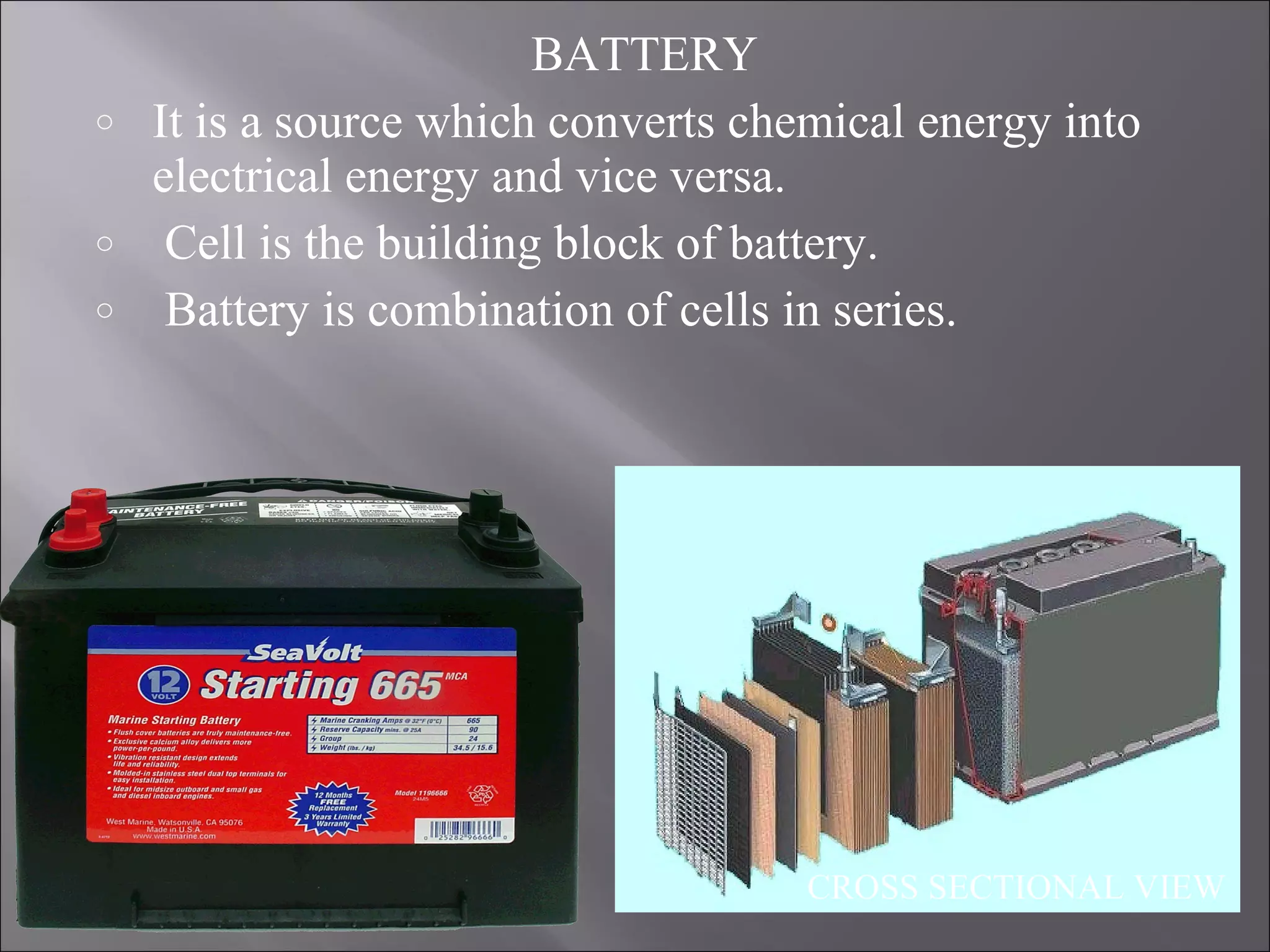
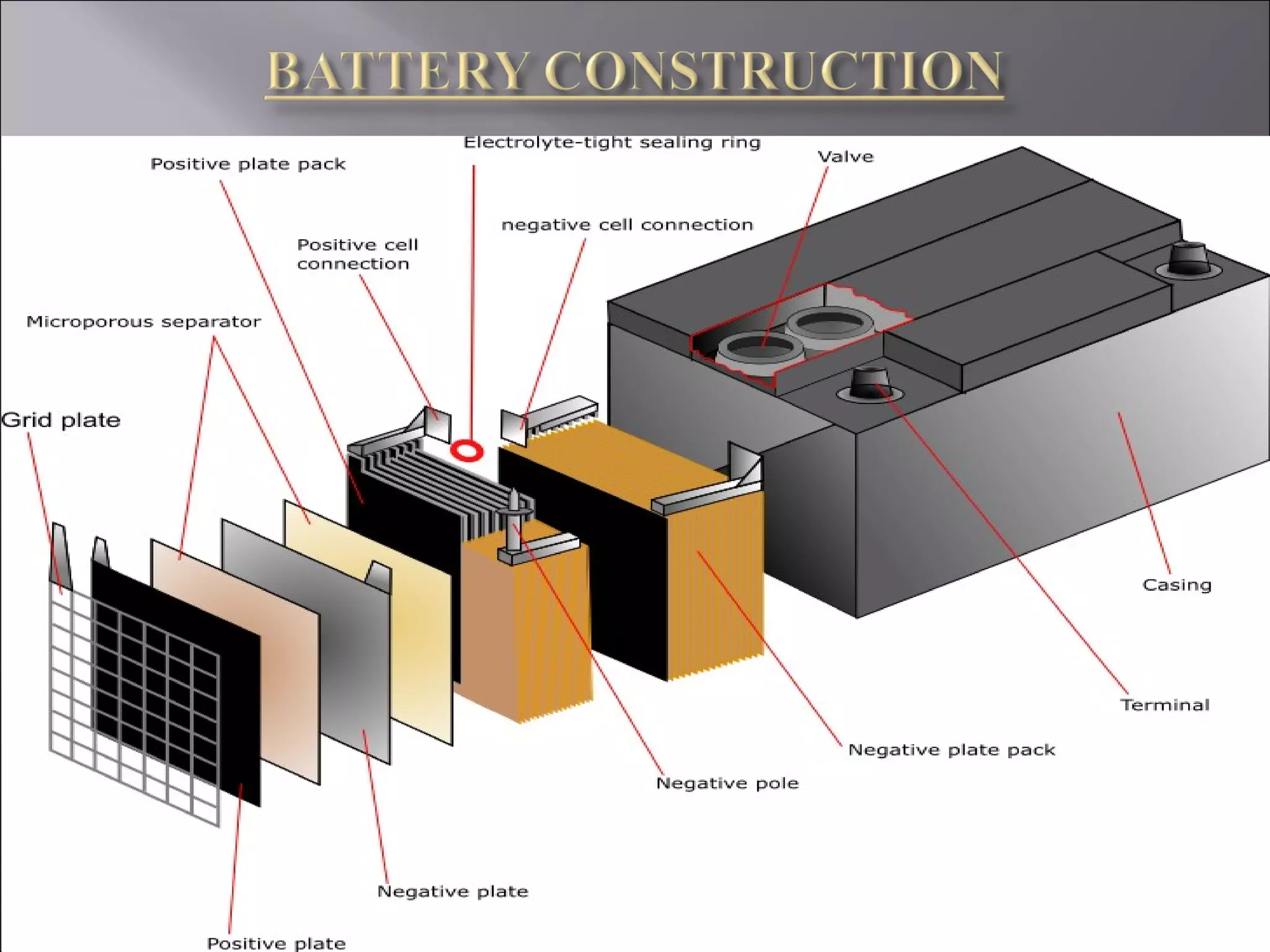
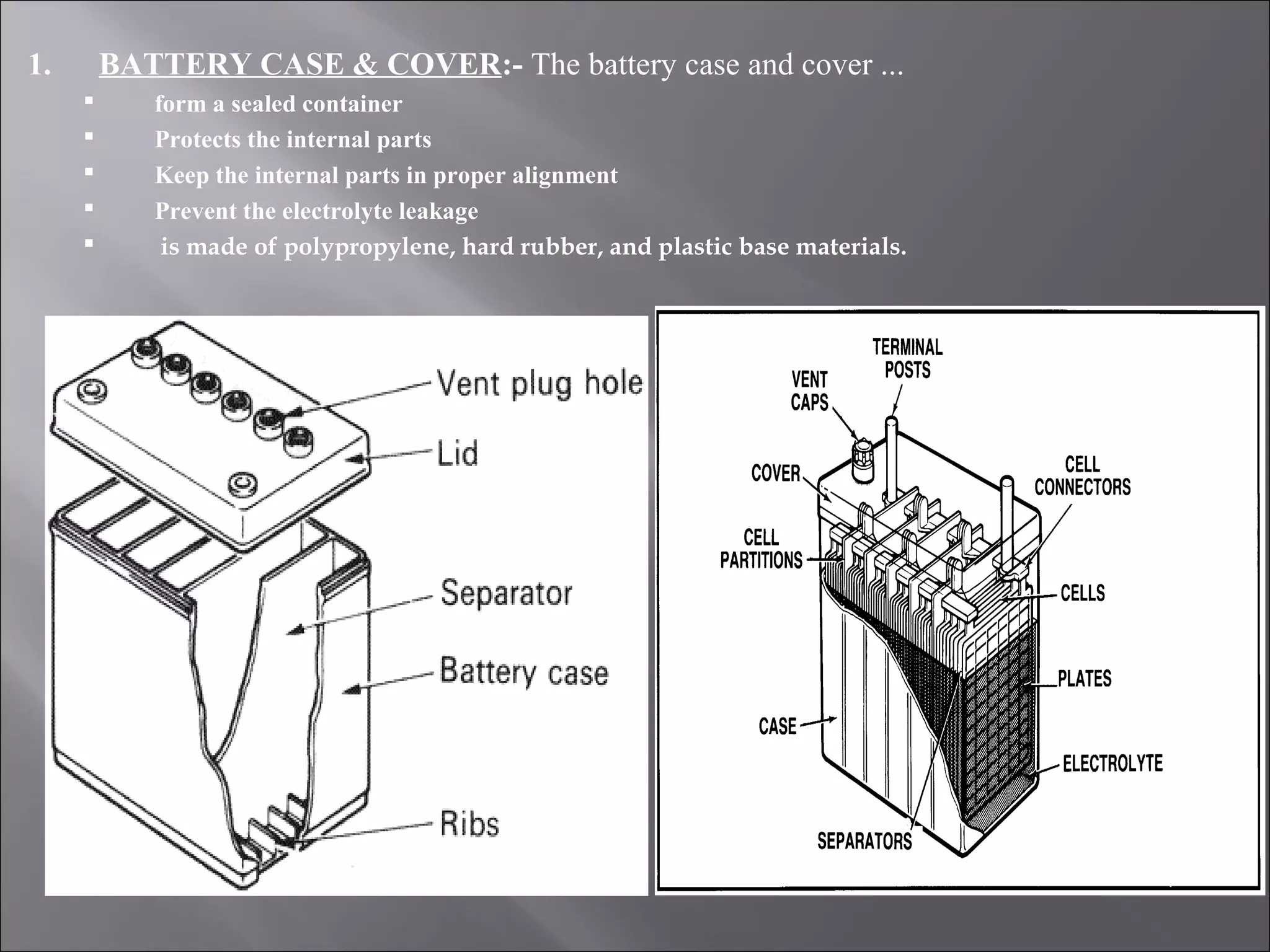
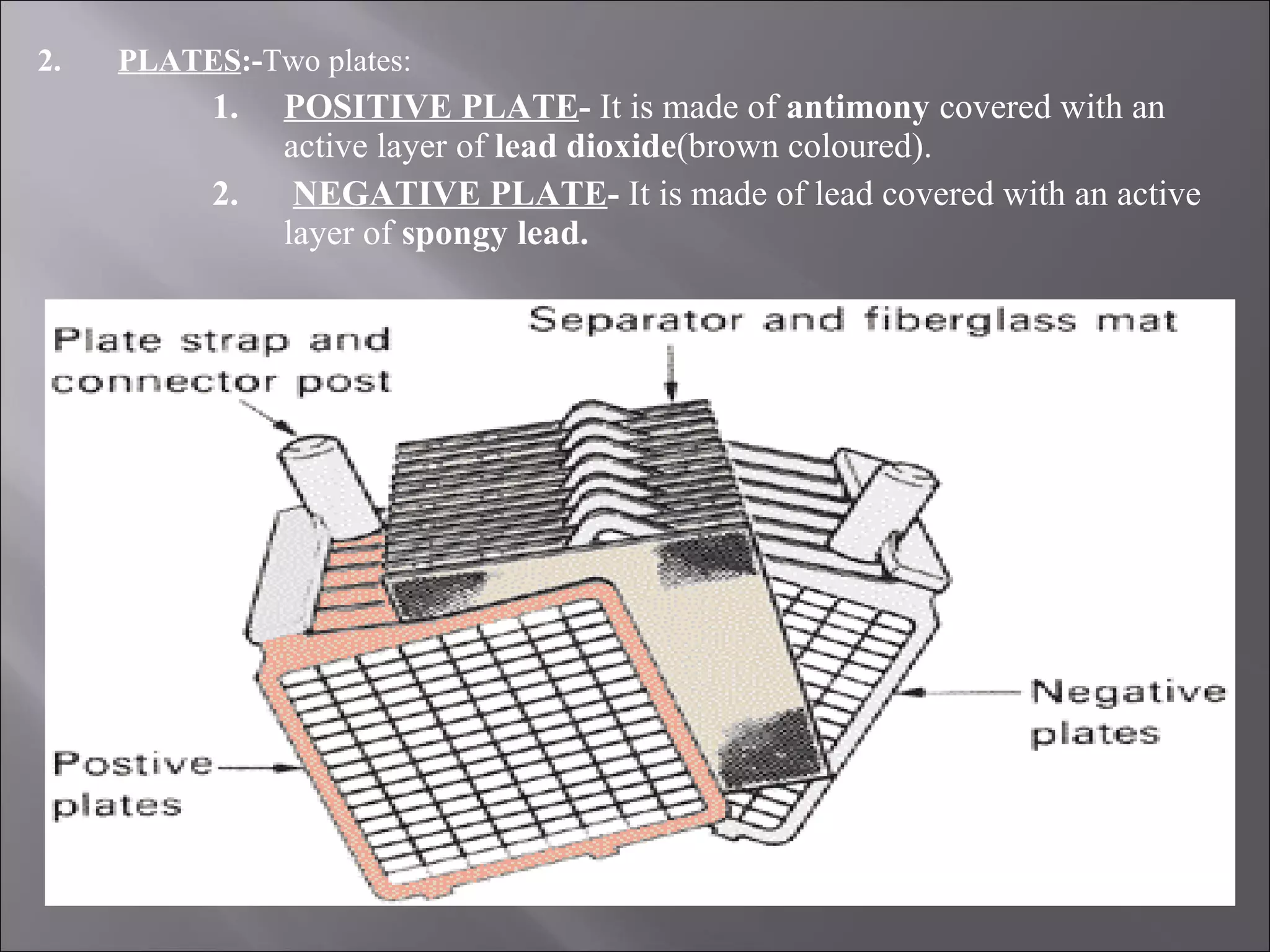
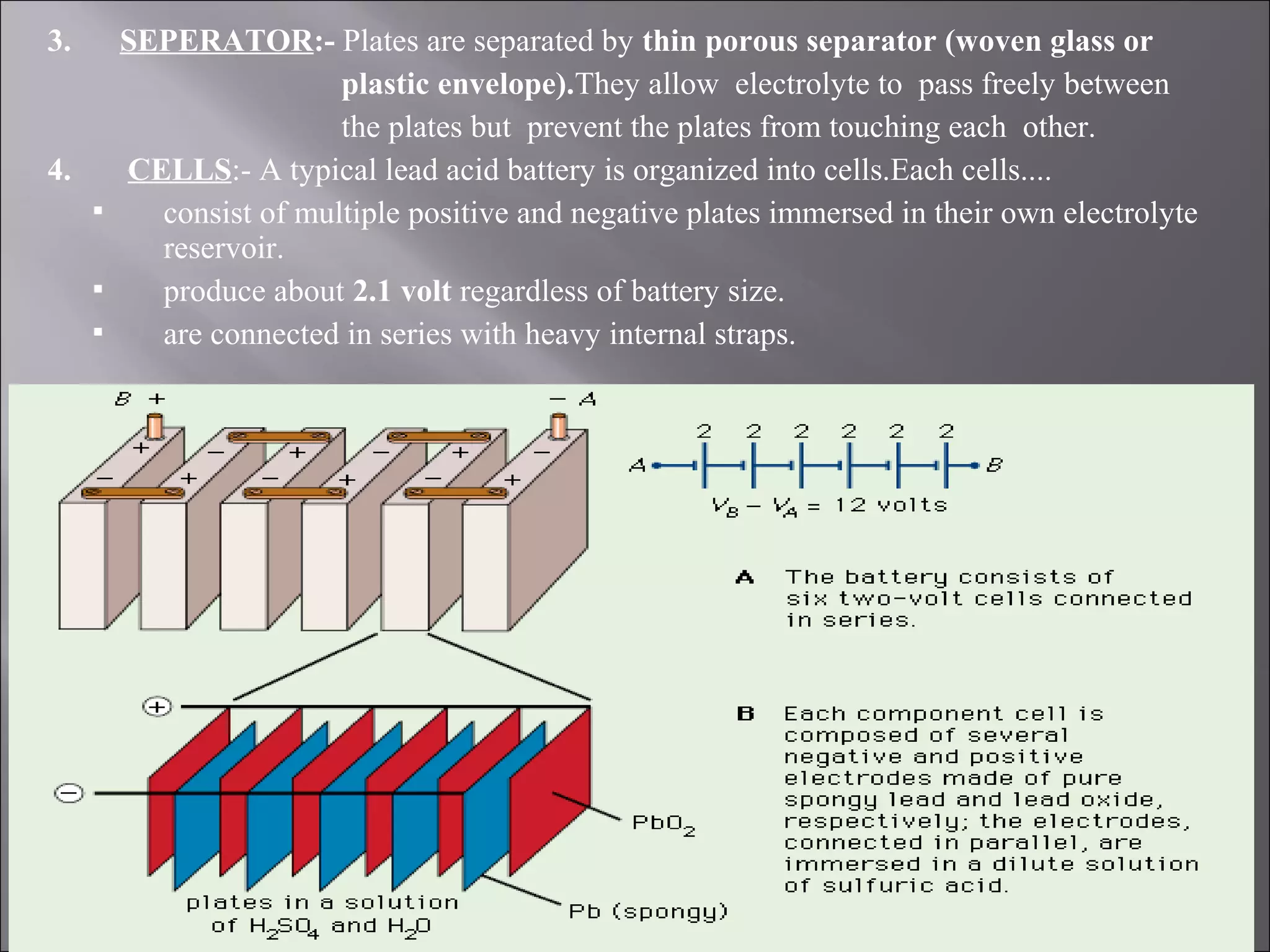
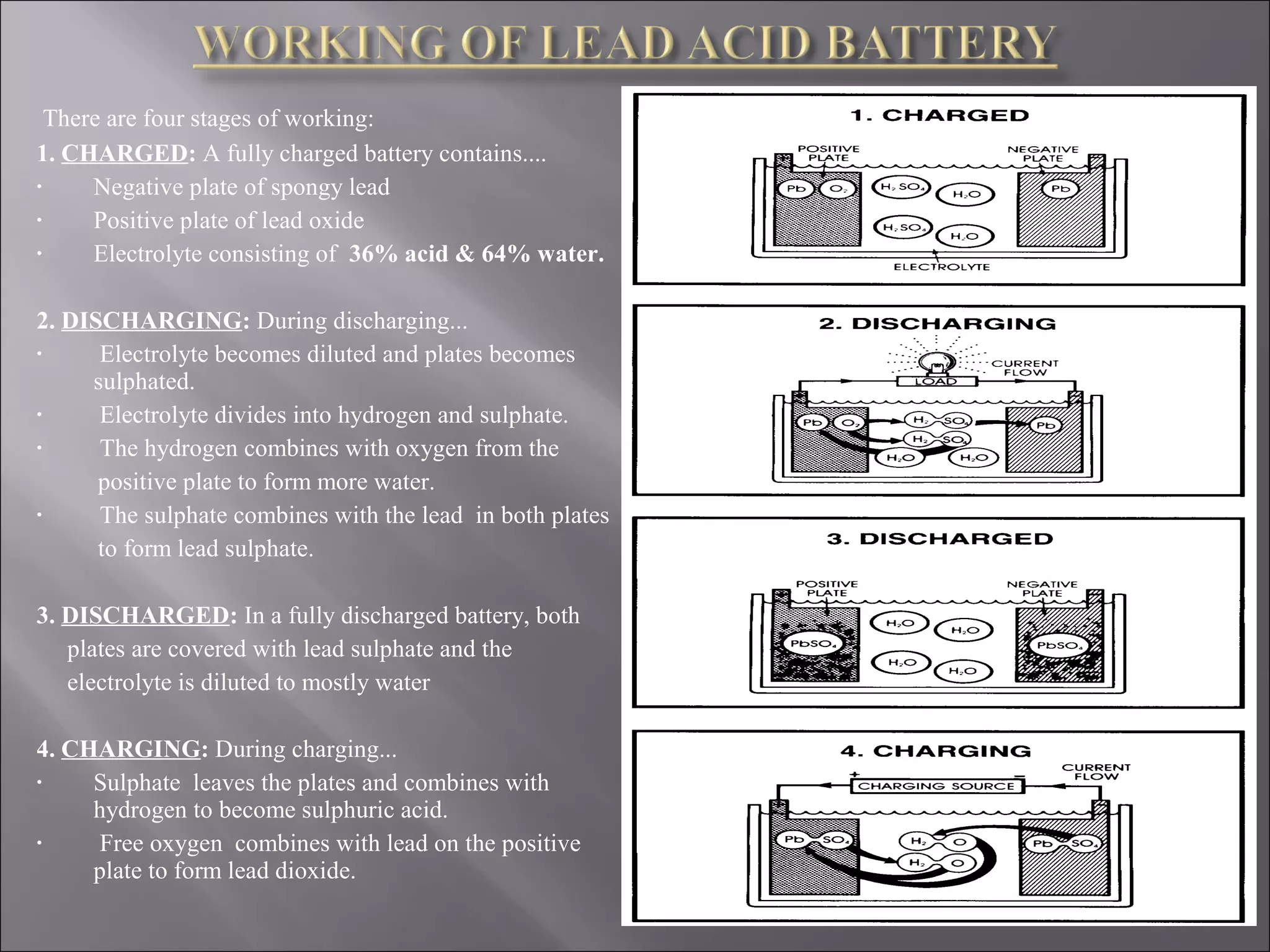
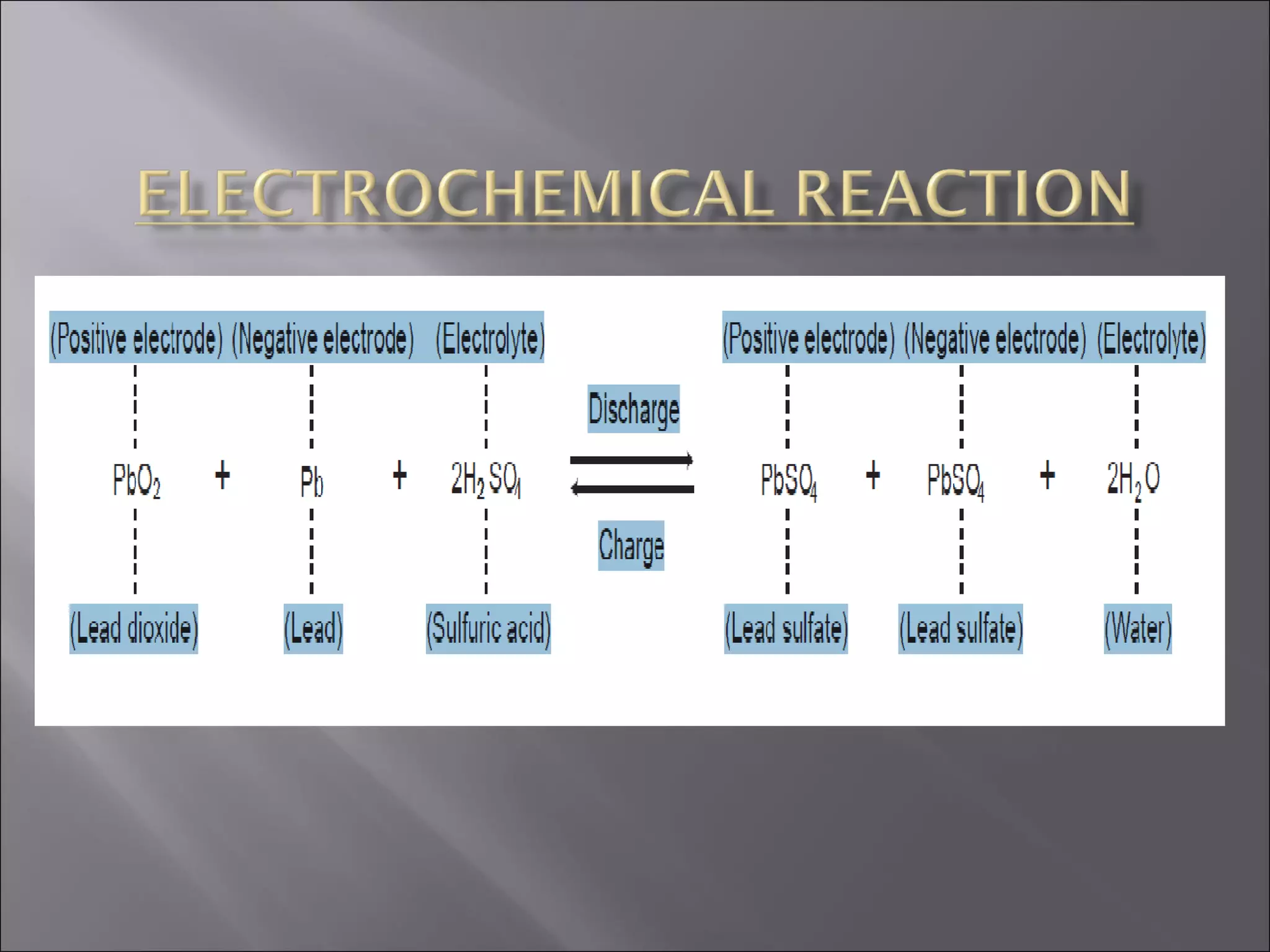
1. The document presents information about lead acid batteries, including their construction, types of cells, and working.
2. A lead acid battery consists of lead plates immersed in sulfuric acid electrolyte within a sealed case. During discharge, the plates convert chemical energy to electrical energy through chemical reactions.
3. The battery works by reversing these chemical reactions during charging, restoring the plates and electrolyte to their original state to allow for further use.