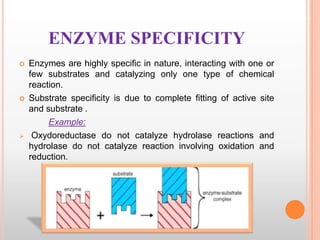
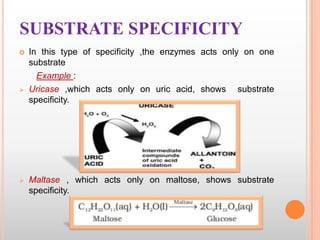
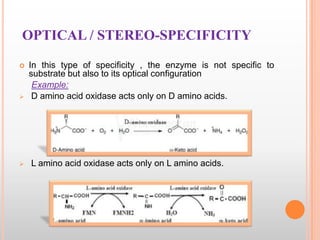
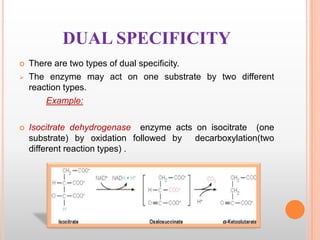
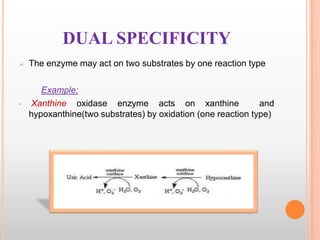
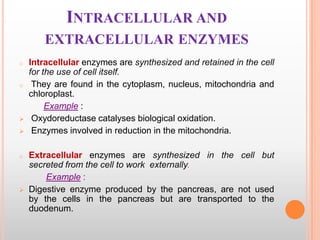
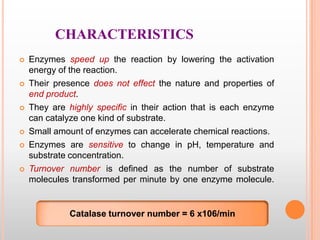
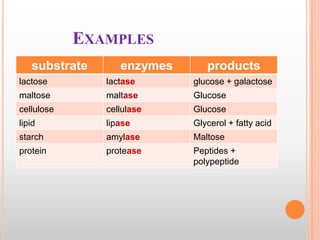
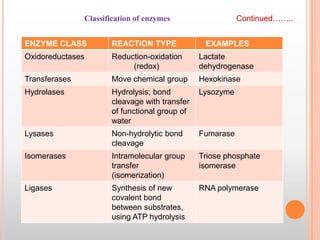
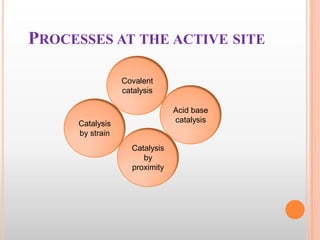
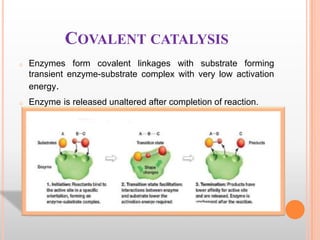
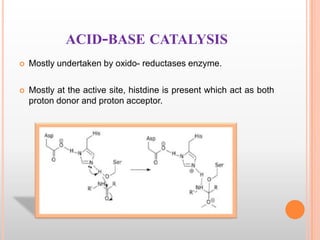
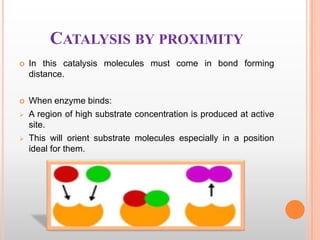
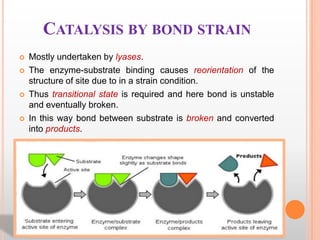
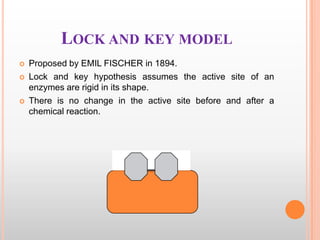
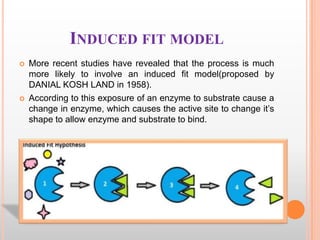
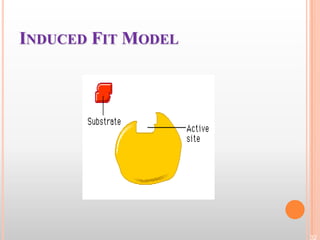
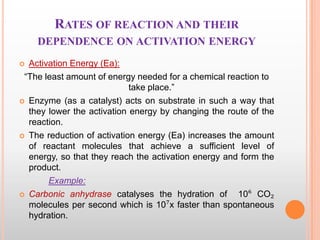
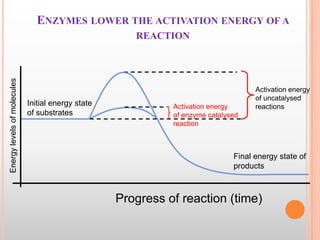
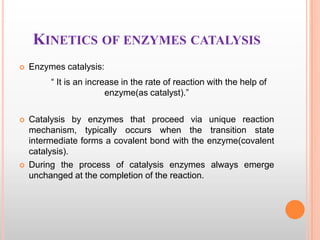
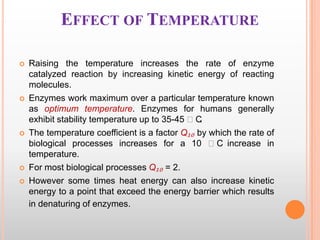
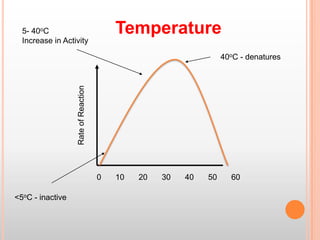
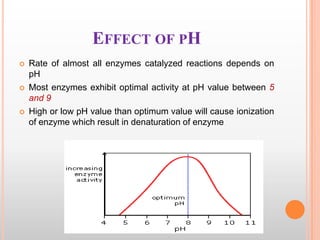
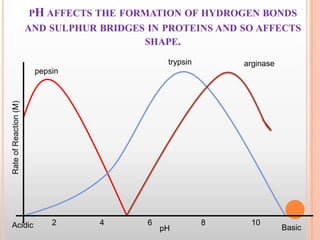
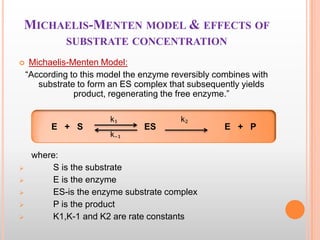
This document provides an overview of enzymes, including their chemistry, classification, mechanisms of action, kinetics, inhibition, and activation. It begins with the basic introduction that enzymes are protein catalysts that speed up biochemical reactions. It then covers enzyme structure and components like cofactors. The major sections explain classification of enzymes based on reaction type, mechanisms like induced fit and catalytic types, kinetics concepts like Michaelis-Menten modeling and factors affecting reaction rates, and types of inhibition like competitive and noncompetitive. The document aims to comprehensively summarize the key topics relating to enzymes.










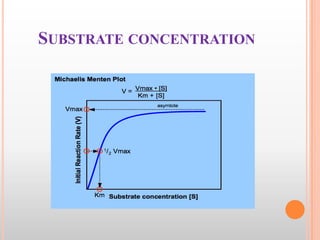
![MICHAELIS-MENTEN EQUATION
Michaelis-Menten Equation:
“It is an equation which describes how reaction velocity varies
with substrate concentration.”
Vmax [S]
Vo=
Km+[S]
Where
Vo is the initial reaction velocity.
Vmax is the maximum velocity.
Km is the Michaelis constant = (k₋₁+k₂)/k₁.
[S] is the substrate concentration.](https://image.slidesharecdn.com/enzymes2-140121084121-phpapp02/85/Enzymes-44-320.jpg)