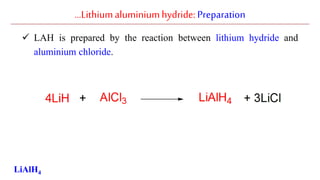
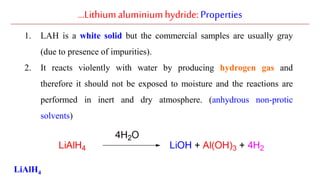
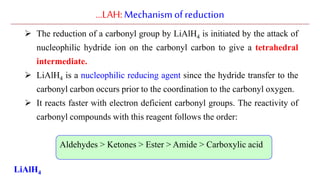
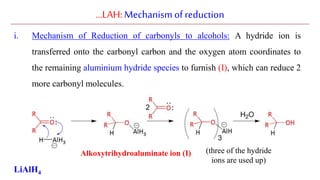
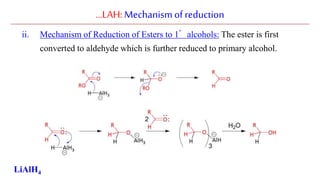
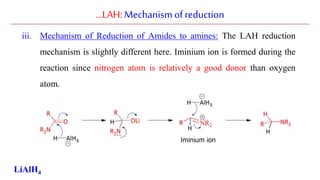
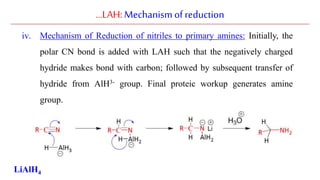
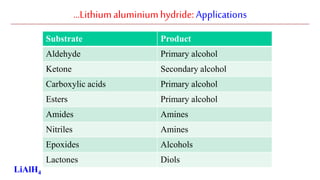
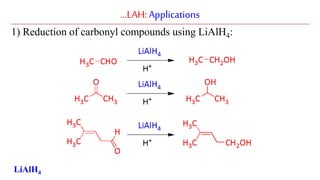
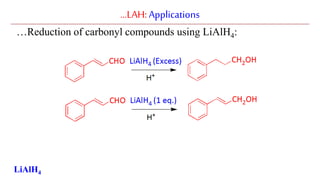
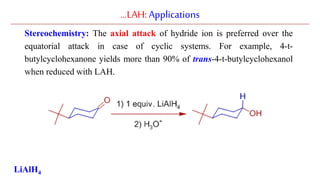
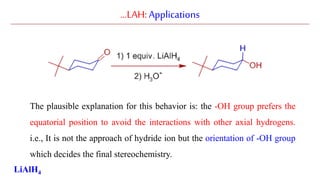
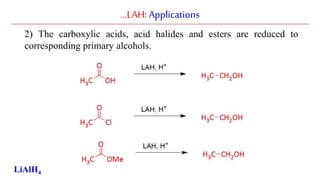
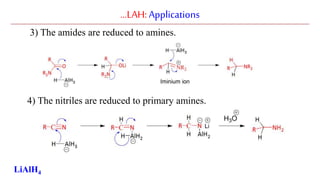
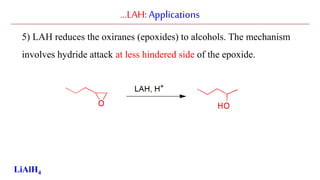
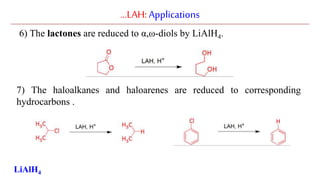
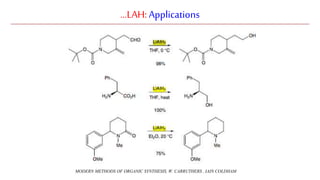
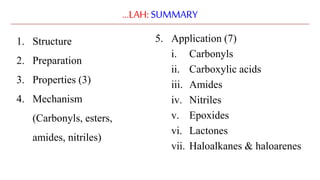
Lithium aluminium hydride (LAH) is a strong reducing agent that is commonly used to reduce carbonyl groups, esters, amides, nitriles, epoxides, lactones, and haloalkanes/haloarenes. LAH is prepared through the reaction of lithium hydride with aluminum chloride. It is a white solid that reacts violently with water, producing hydrogen gas, so reactions must be performed under anhydrous conditions. The mechanism of LAH involves nucleophilic hydride attack on the carbonyl carbon to form an intermediate tetrahedral structure.