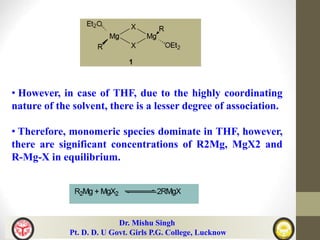
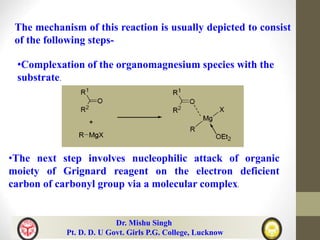
The document discusses organomagnesium halides (Grignard reagents). It describes how Grignard reagents are useful synthetic intermediates for carbon-carbon bond formation and are sources of nucleophilic carbanions. It summarizes several reactions of Grignard reagents including additions to carbonyl compounds, substitutions of alkyl/alkenyl/aryl halides, synthesis of ethers, amines, aldehydes and more. It also discusses the mechanisms and applications of important Grignard reactions such as the Bouveault, Benary, Bodrous, Dzhemilev and Kulinkovich reactions.