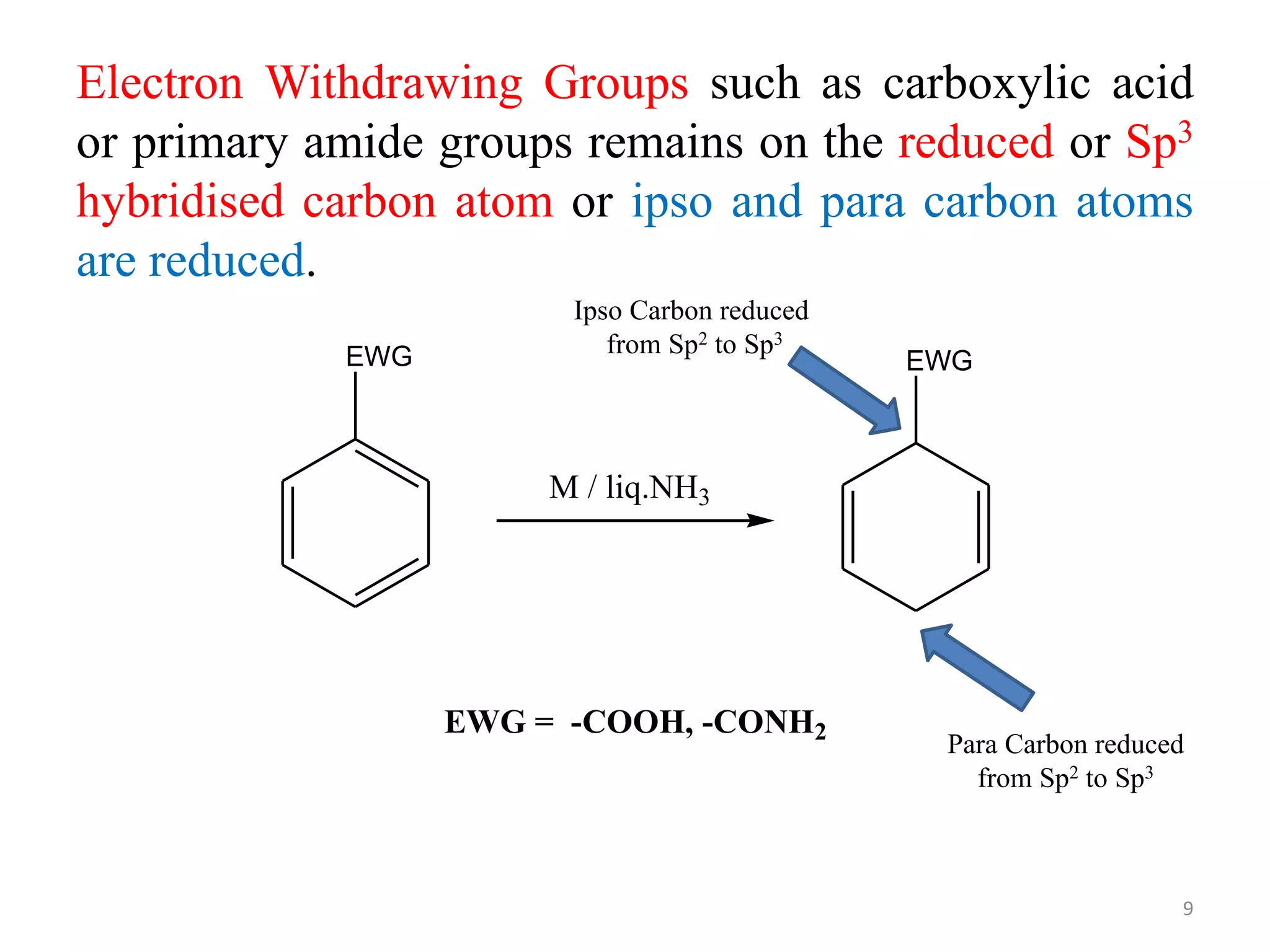
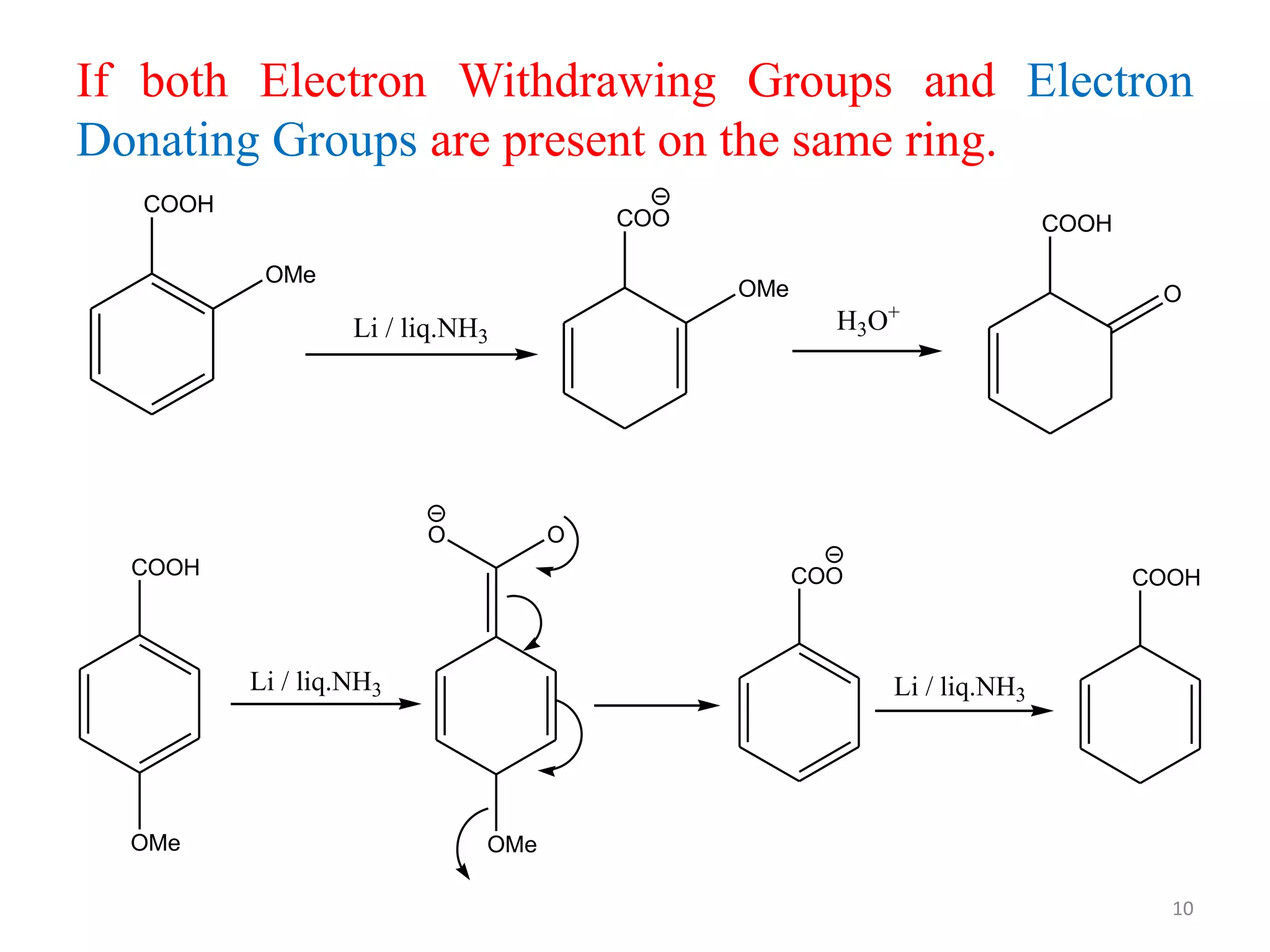
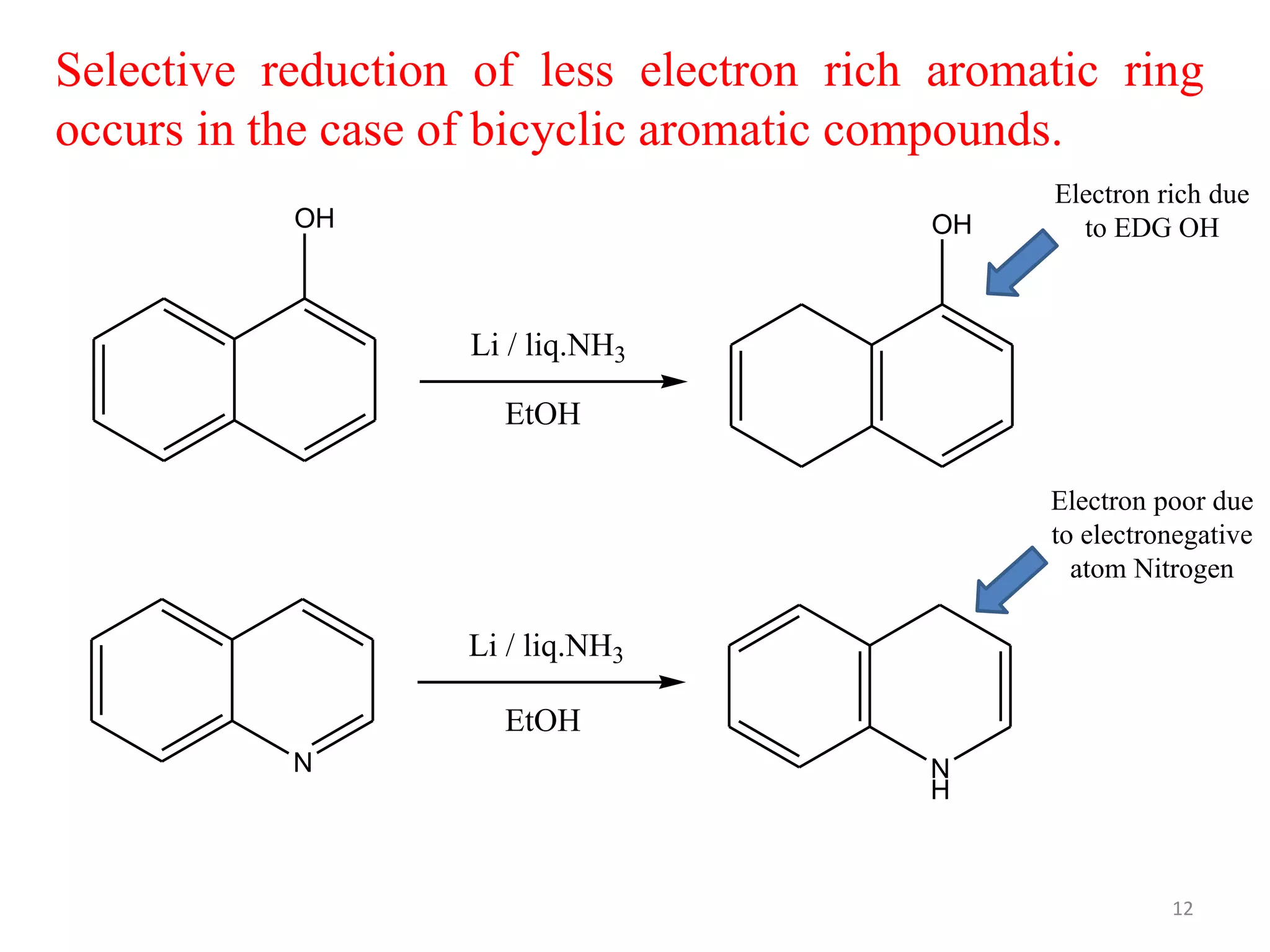
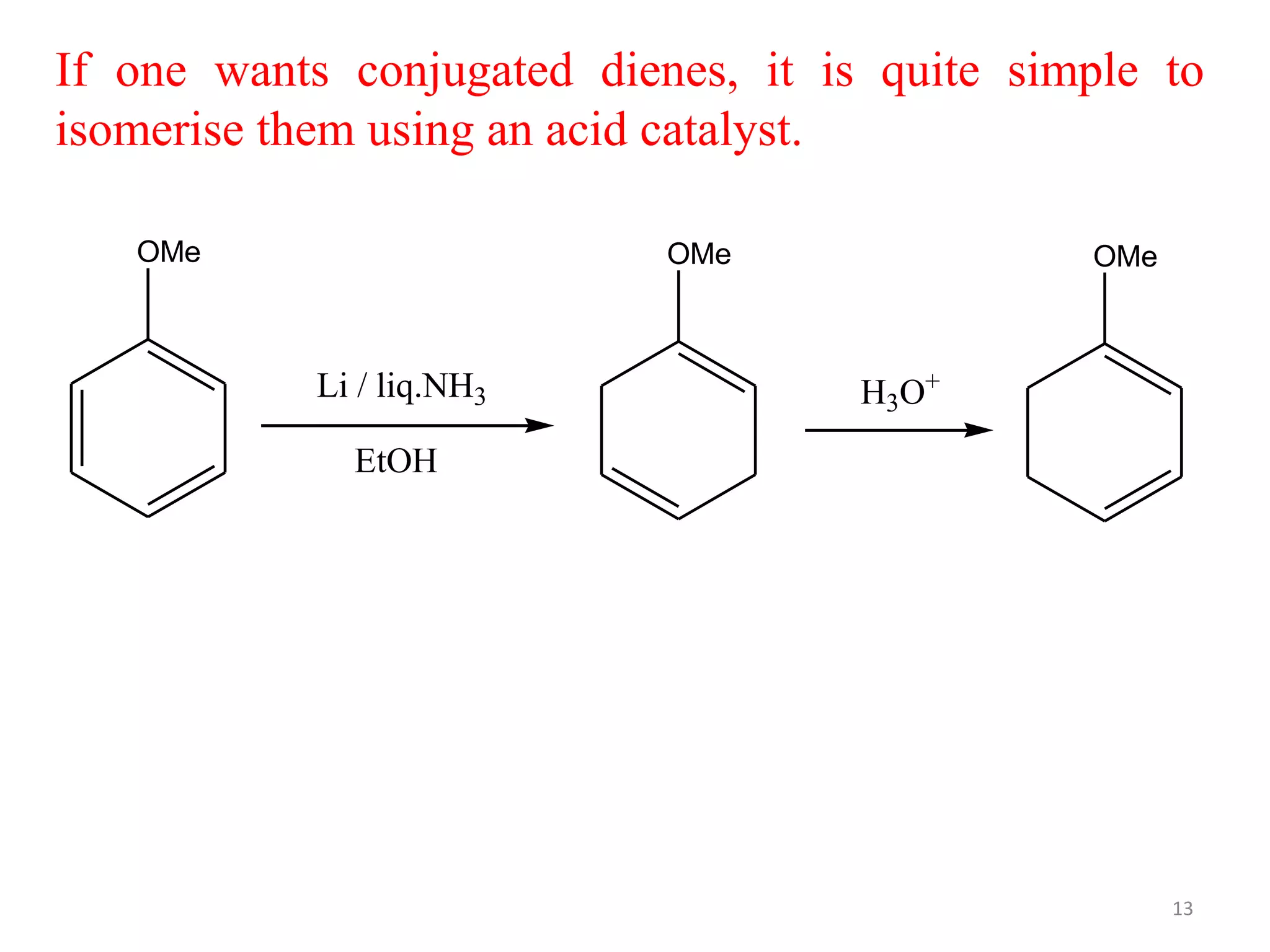
The Birch reduction is a reaction where aromatic compounds undergo partial reduction to unconjugated cyclohexadiene compounds in the presence of alkali metals like sodium or lithium in liquid ammonia. The solvated electrons from the reaction of the metal with liquid ammonia give the solution an intense blue color. The mechanism begins with single electron transfer from the metal to the aromatic ring, forming a radical anion. Regioselectivity in the reduction depends on whether substituents on the aromatic ring are electron donating groups or electron withdrawing groups. The Birch reduction can selectively reduce the less electron-rich ring in bicyclic aromatic compounds.


![The Birch reduction is an organic reaction where
aromatic compounds undergo partial reduction to 1,4-
unconjugated cyclohexadiene compounds in presence of
alkali metals in liquid ammonia i.e. solvated electrons.
M liq. NH3 M [H3N-------e-------NH3]
M = Na / Li (Solvated Electron)
H H
H H
M / Liq. NH3
(Aromatic) (Non-aromatic)
M = Na / Li
The reduction is conducted by Sodium or Lithium metal
in liquid ammonia at -33oC
3](https://image.slidesharecdn.com/birchreduction-191129181218/75/Birch-reduction-3-2048.jpg)
![The solvated electrons give an intense blue color to the
solution and have to be captured as the metal releases
them, otherwise with time the blue color fades as the
electrons reduce the ammonia to NH2
- and H2.
Li Li e [NH3]n
NH3 e NH2 H
1/2 H2
(Blue solution)
(Colorless solution)
4](https://image.slidesharecdn.com/birchreduction-191129181218/75/Birch-reduction-4-2048.jpg)