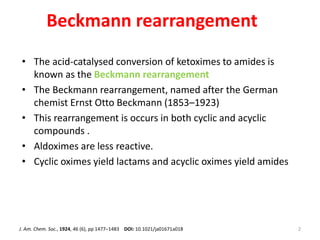
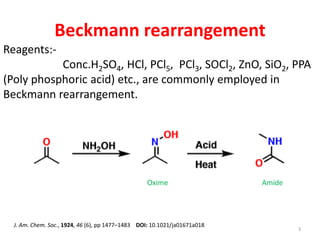
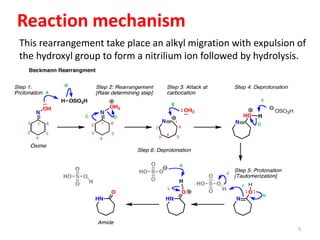
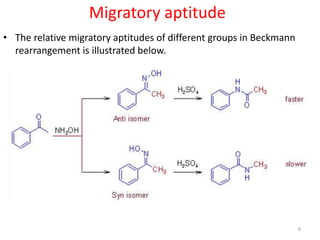
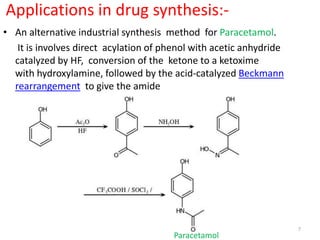
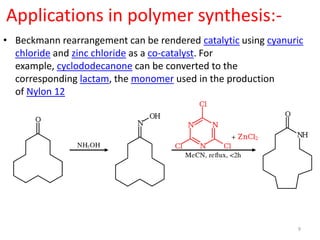
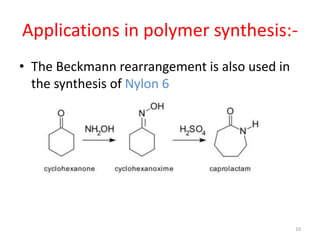
The Beckmann rearrangement is an acid-catalyzed reaction that converts ketoximes to amides. It was discovered by German chemist Ernst Otto Beckmann in the late 19th century. This rearrangement can occur in both cyclic and acyclic compounds, converting ketoximes to lactams or amides, respectively. Common reagents used to catalyze the Beckmann rearrangement include concentrated sulfuric acid, hydrochloric acid, and phosphorus pentachloride. The reaction proceeds through the formation of a nitrilium ion intermediate followed by hydrolysis to form the final amide product. The Beckmann rearrangement has applications in synthesizing drugs like paracetamol and polymers like nylon.