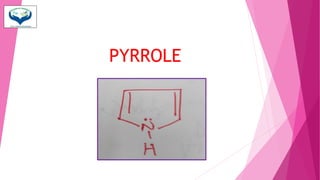
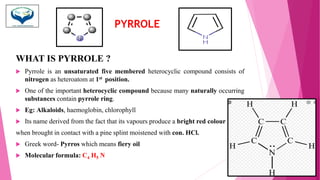
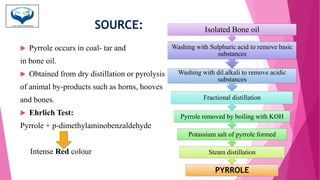
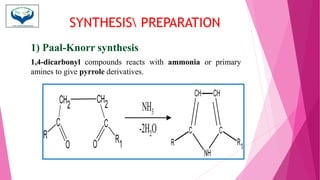
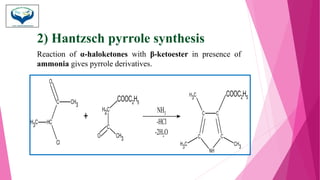
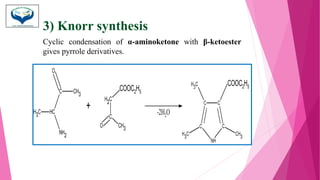
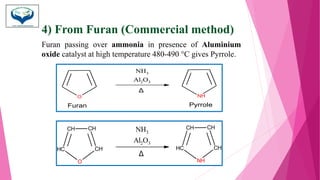
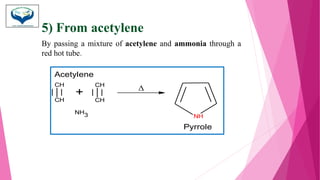
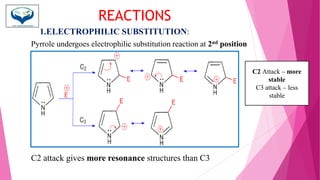
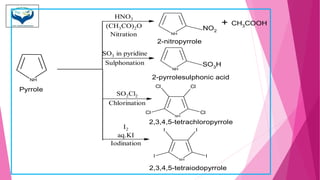
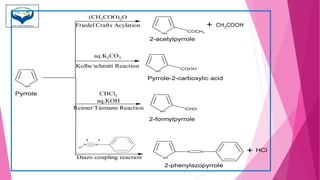
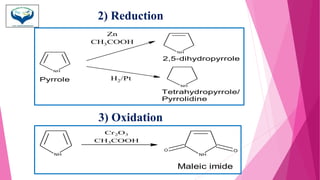
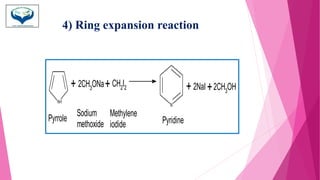
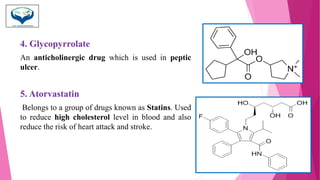
This document discusses the heterocyclic compound pyrrole. It begins by defining pyrrole as an unsaturated five-membered ring containing nitrogen. Pyrrole is an important compound found naturally in substances like alkaloids, hemoglobin, and chlorophyll. The document then describes several methods for synthesizing pyrrole, including the Paal-Knorr, Hantzsch, and Knorr syntheses. It also discusses some reactions pyrrole undergoes, such as electrophilic substitution and reduction. Finally, it lists several medicinal uses of pyrrole derivatives, including the amino acid proline, the stimulant nicotine, and drugs used to treat Parkinson's disease and peptic