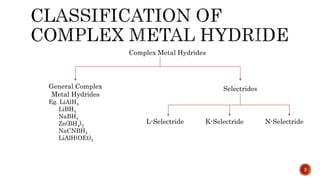
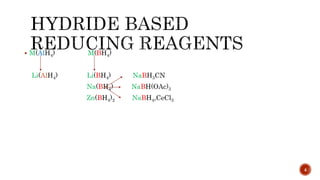
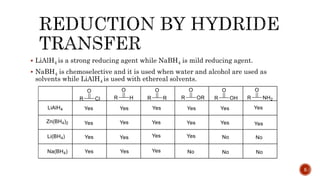
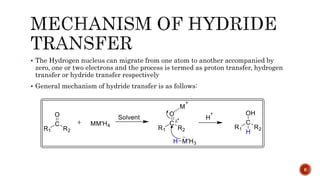
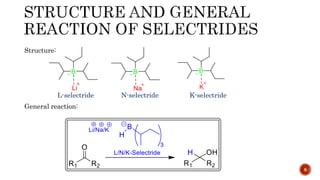
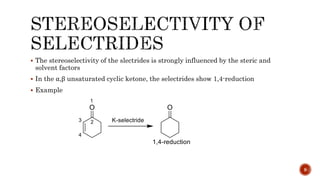
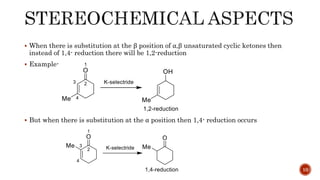
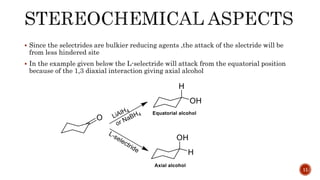
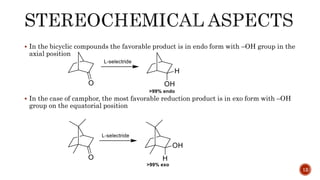
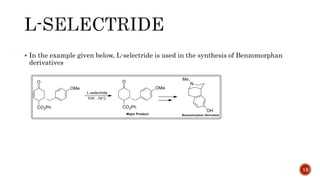
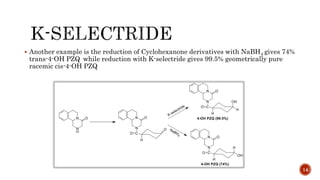
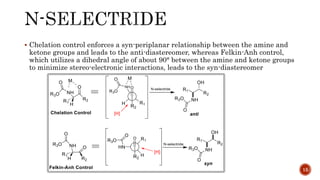
The document discusses complex metal hydrides, characterized by the general formula mxm'yhn, and provides examples and properties of various types. It details the role of these hydrides as nucleophilic sources and their reactivity towards electrophiles, as well as the specific behaviors of selectrides, which influence stereoselectivity in reactions. Furthermore, the document highlights the importance of steric and solvent factors in dictating the mechanisms of hydride transfer and reduction processes.