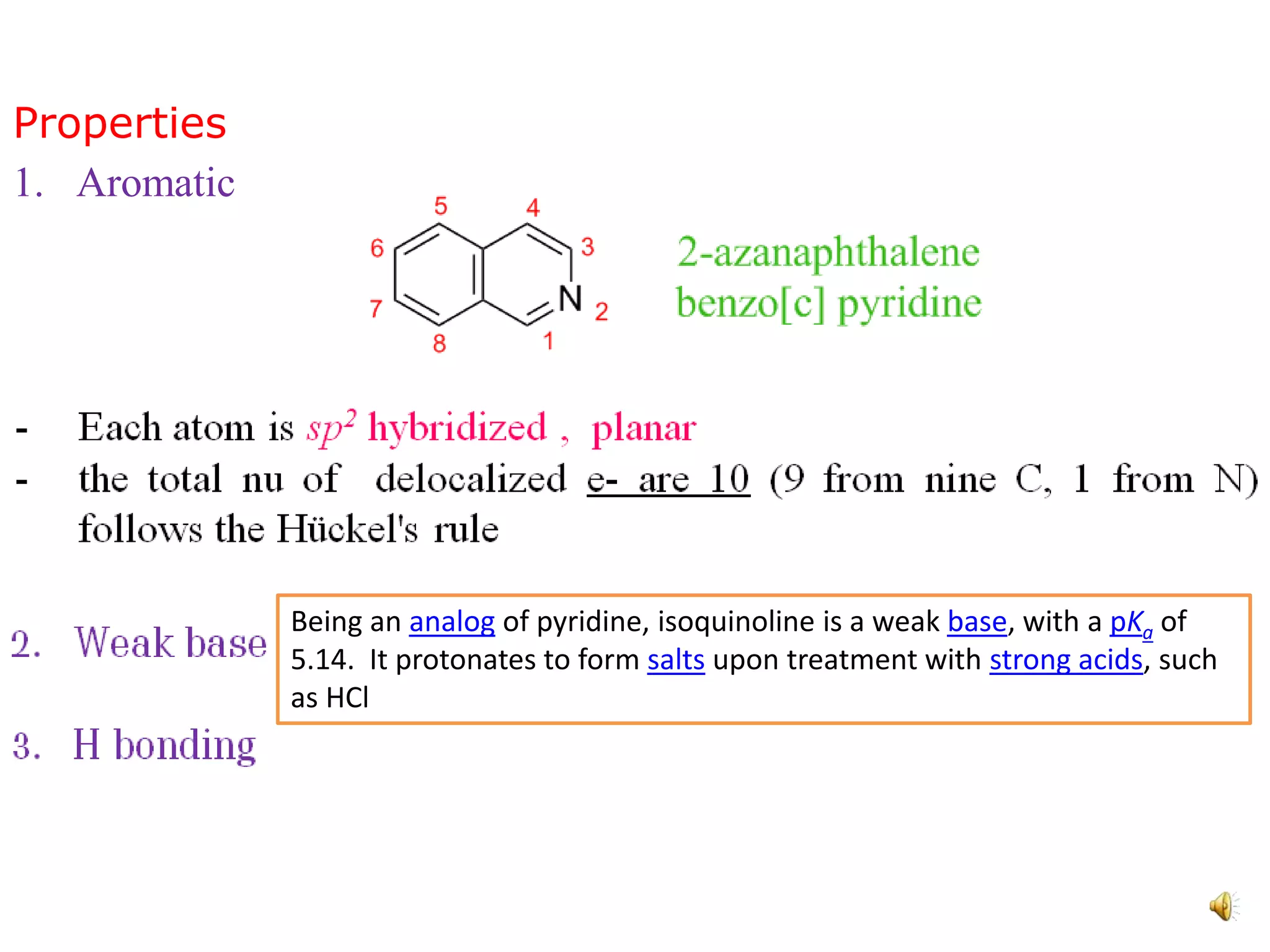
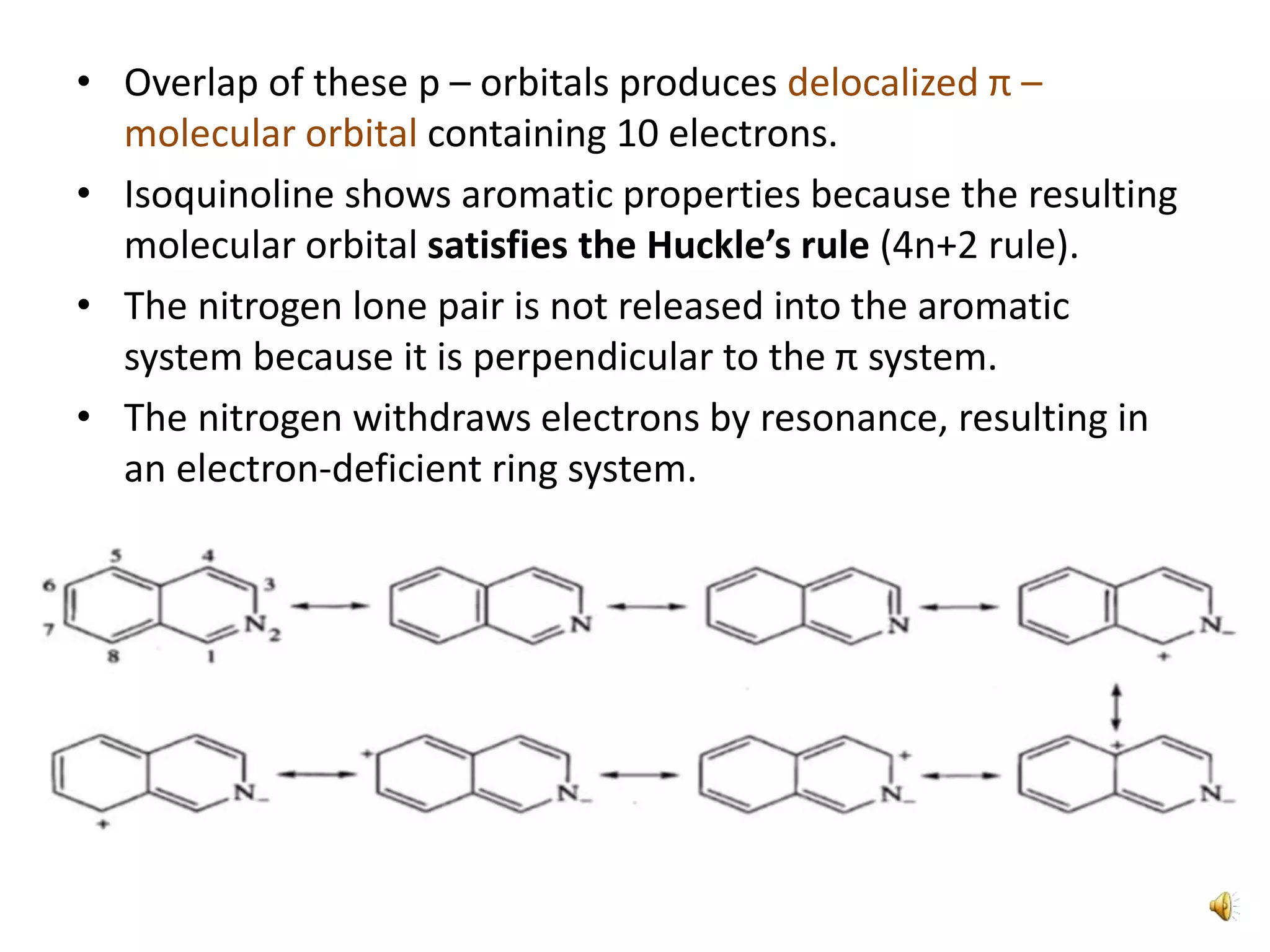
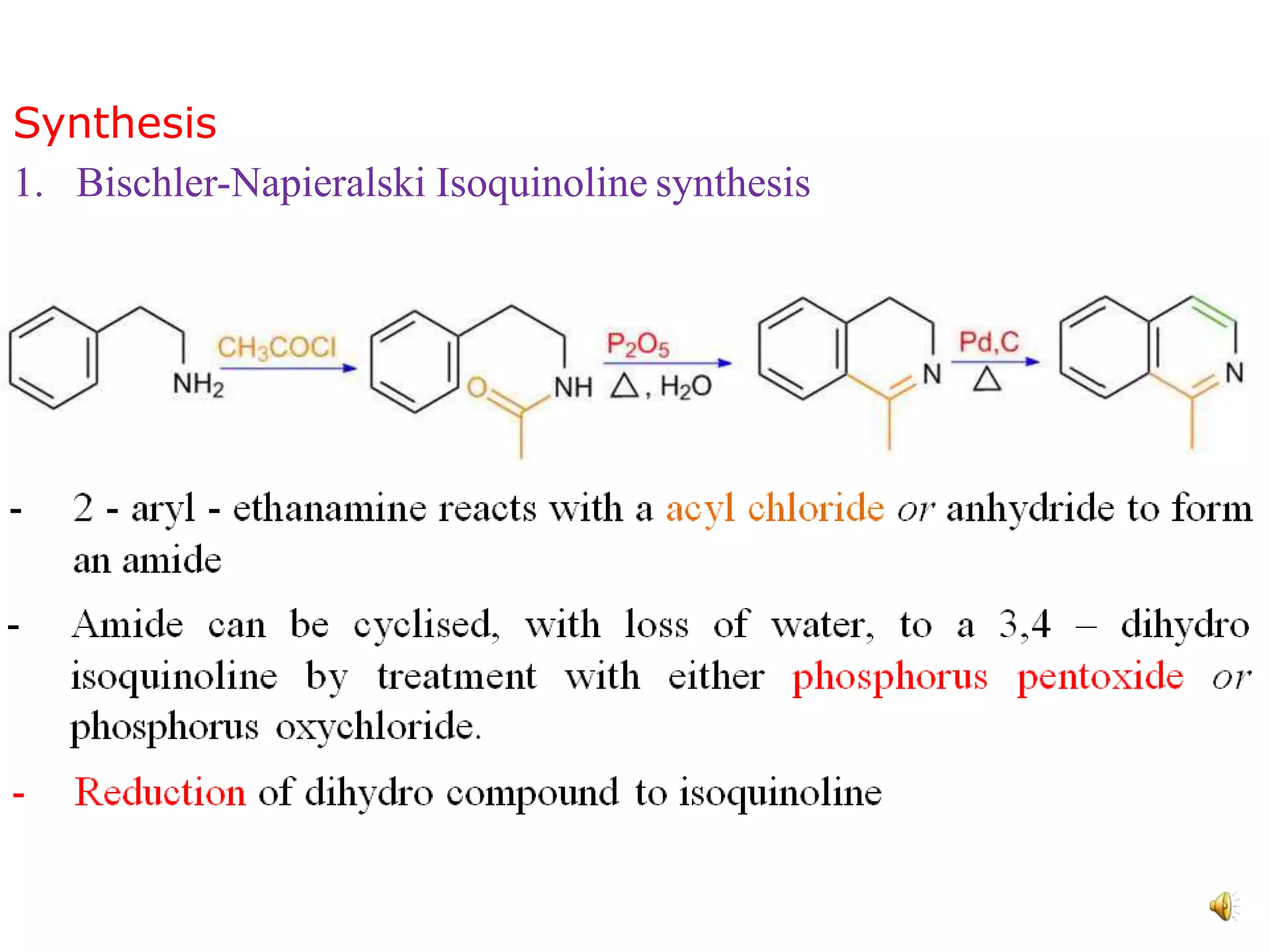
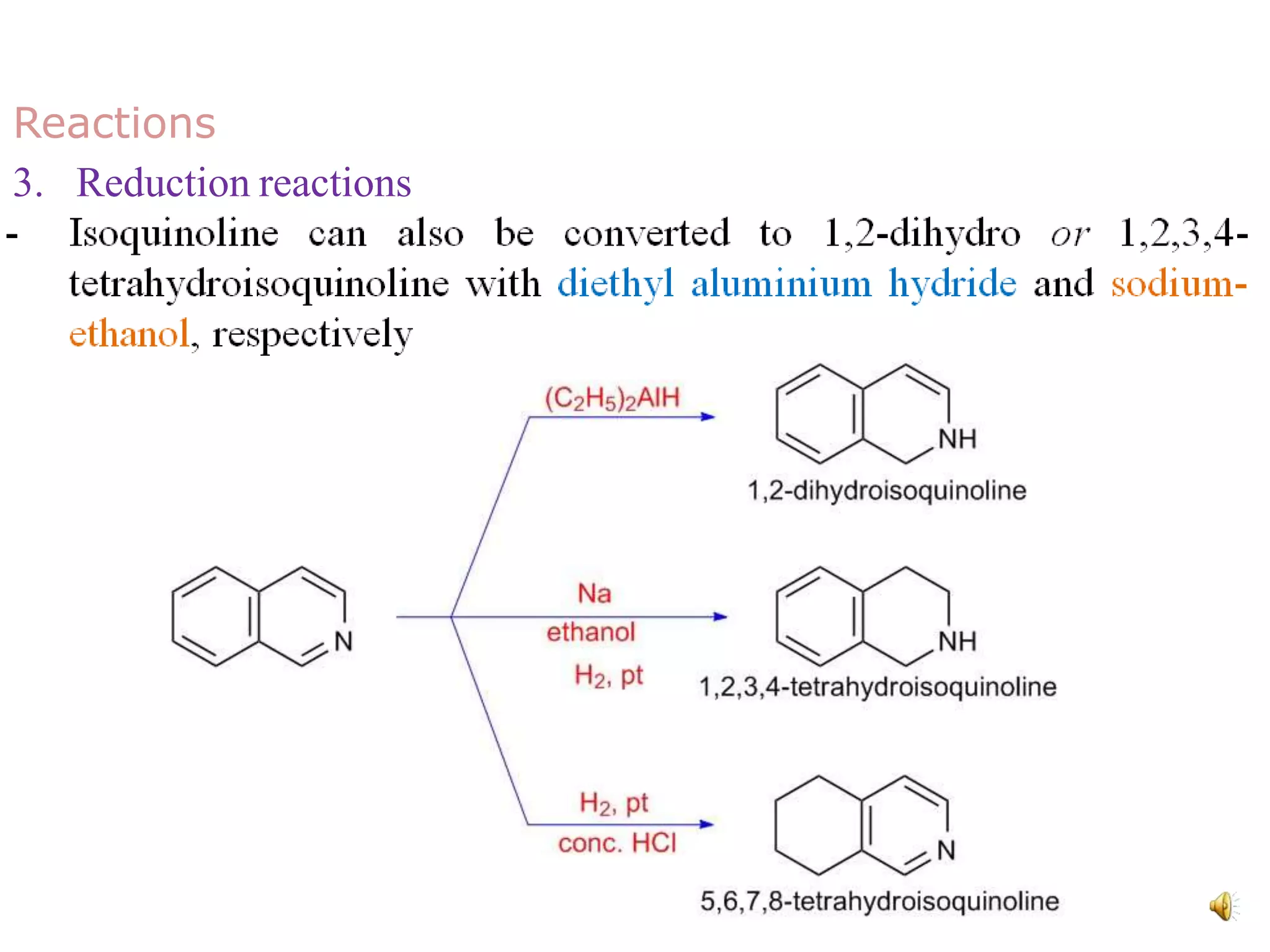
Isoquinoline is a heterocyclic aromatic organic compound and structural isomer of quinoline, first isolated from coal tar in 1885. It possesses unique physical properties, including being a colorless hygroscopic liquid with a low solubility in water, and serves as a structural backbone in naturally occurring alkaloids. Isoquinoline has various medicinal uses, including applications in treating high blood pressure and as an anesthetic.

![Isoquinoline is a heterocyclic aromatic organic
compound. It is a structural isomer of quinoline.
Isoquinoline and quinoline are benzopyridines,
which are composed of a benzene ring fused to
a pyridine ring.
Other names
Benzo[c]pyridine
2-benzazine](https://image.slidesharecdn.com/fusedheterocycliccompound-isoquinoline-200630134238/75/Fused-heterocyclic-compound-isoquinoline-2-2048.jpg)