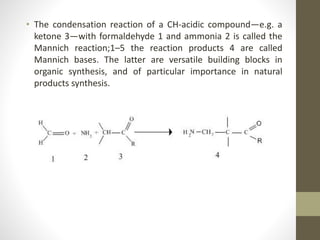
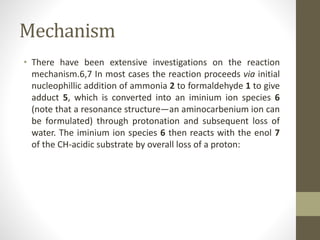
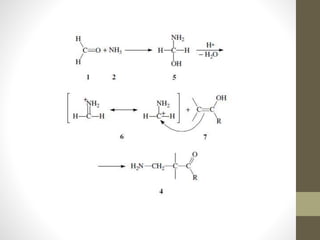
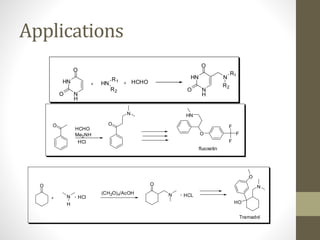
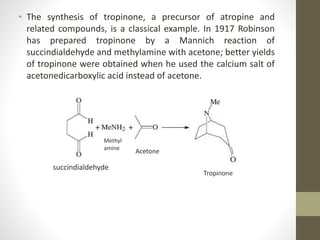
The Mannich reaction involves the condensation of an enolizable carbonyl compound, an aldehyde such as formaldehyde, and an amine to form a β-amino carbonyl compound known as a Mannich base. The reaction proceeds via the initial addition of the amine to the aldehyde to form an iminium ion intermediate, which then reacts with the enol form of the carbonyl compound to eliminate a proton and form the Mannich base product. While versatile building blocks in organic synthesis, the Mannich reaction has limitations in terms of substrate scope and control of regio- and stereoselectivity. Examples of applications include the synthesis of tropinone, a precursor of atropine, as well