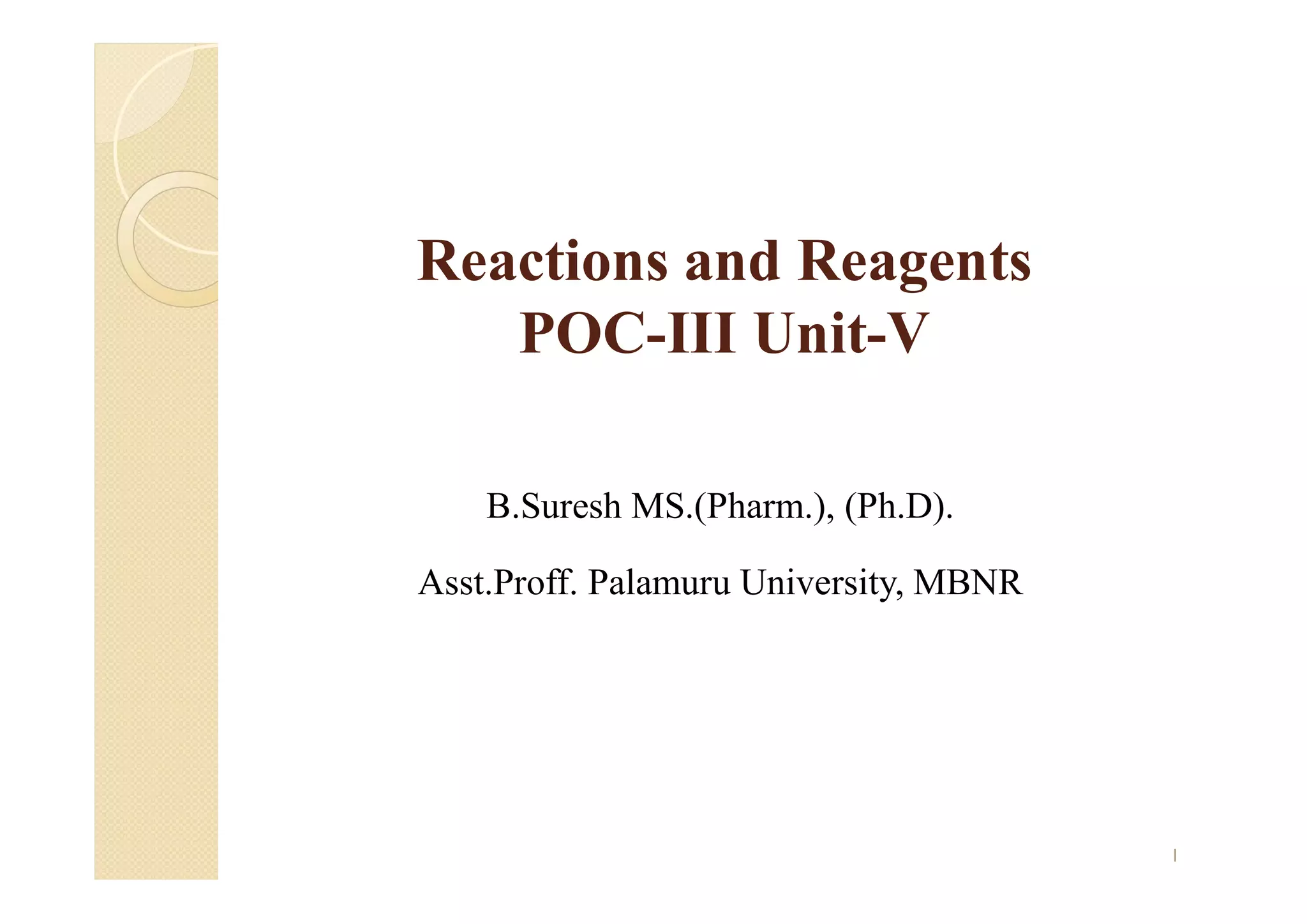
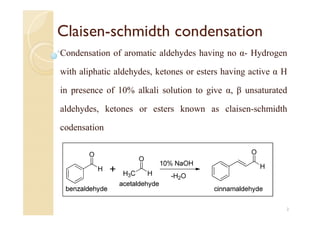
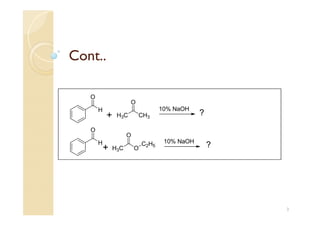
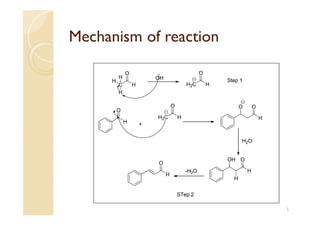
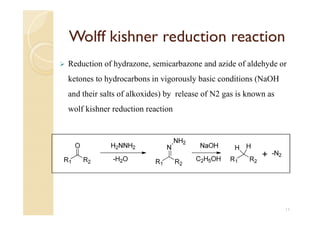
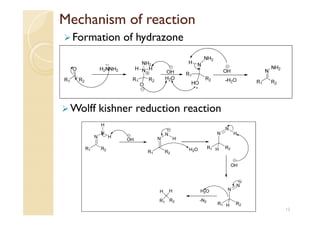
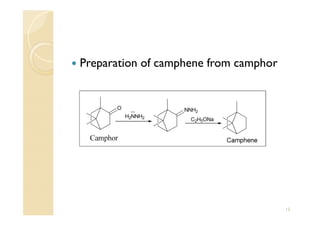
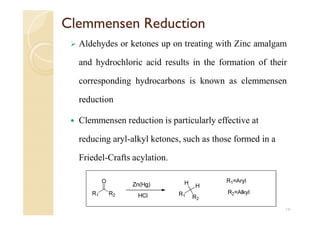
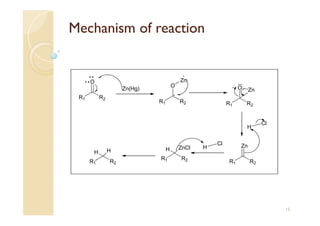
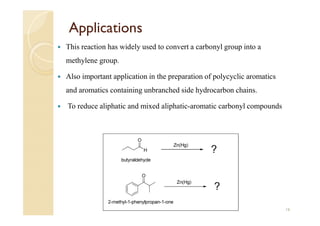
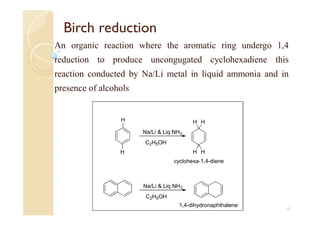
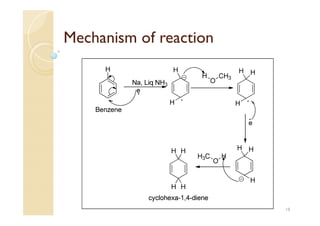
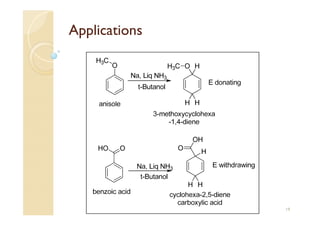
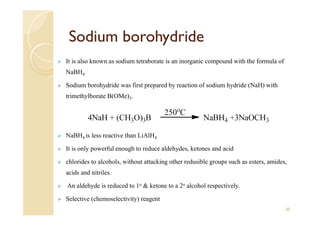
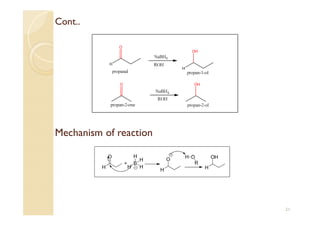
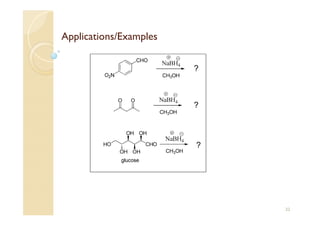
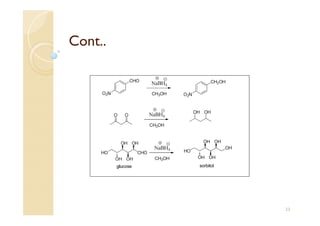
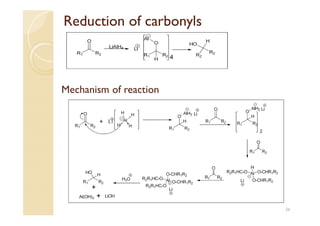
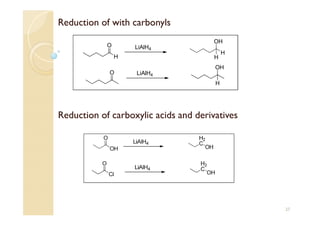
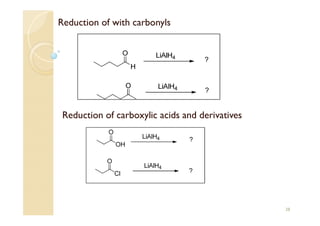
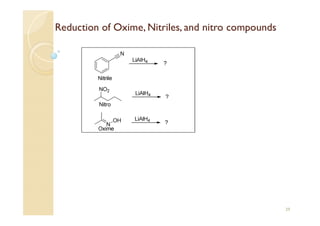
1) The Claisen-Schmidt condensation involves the condensation of aromatic aldehydes without alpha hydrogens and aliphatic aldehydes, ketones, or esters with active alpha hydrogens in the presence of alkaline solutions to form alpha, beta unsaturated aldehydes, ketones, or esters.
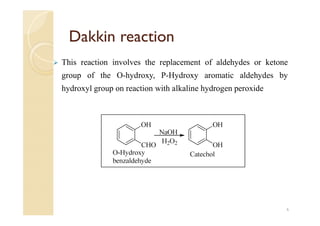
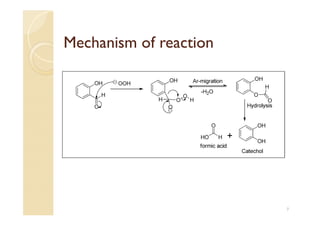
2) The Dakin reaction involves the replacement of aldehyde or ketone groups on O-hydroxy or P-hydroxy aromatic aldehydes by hydroxyl groups when reacted with alkaline hydrogen peroxide.
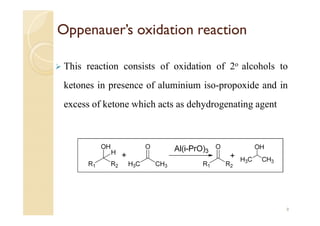
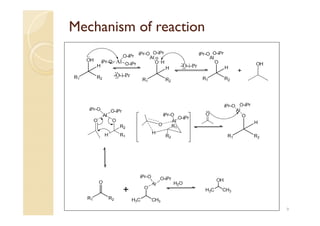
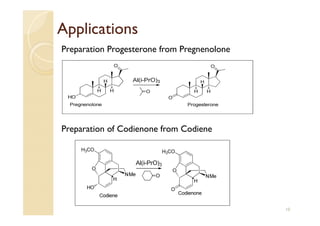
3) The Oppenauer oxidation reaction consists of oxidizing secondary alcohols to ketones in the presence of aluminum isopropoxide and excess ketone which