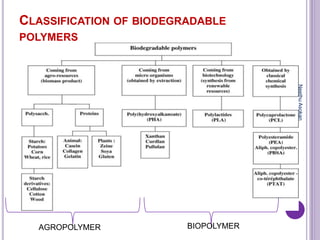
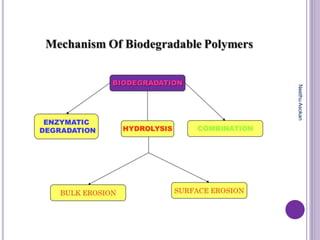
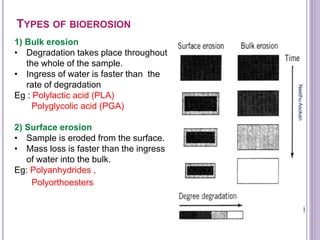
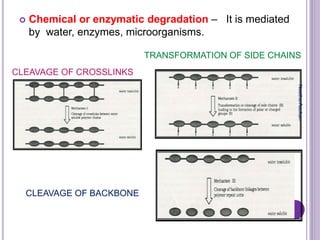
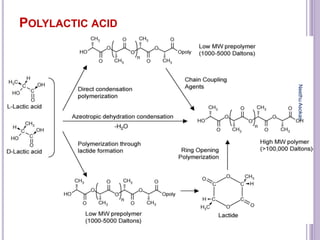
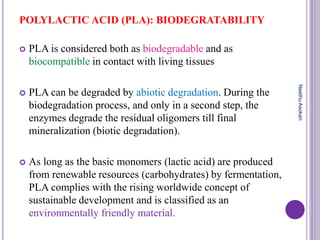
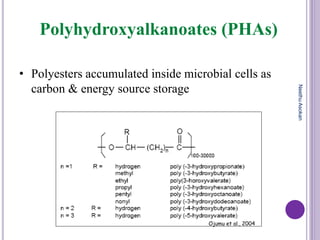
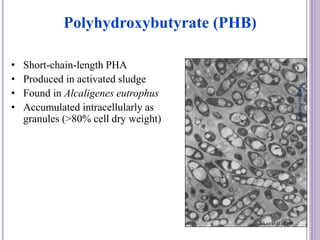
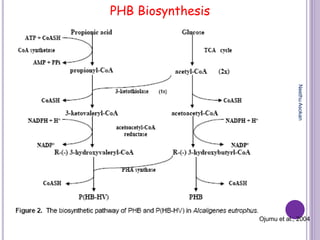
The document discusses biopolymers, which are polymers produced by living organisms. It covers various types of biodegradable polymers including synthetic polymers like polylactic acid (PLA) and natural polymers like starch. The mechanisms of polymer biodegradation are described. Applications of biodegradable polymers in areas like biomedical, packaging and agriculture are also mentioned. Factors affecting the biodegradation of polymers are discussed. Current trends in biopolymers including their use as alternatives to petroleum-based plastics are summarized.