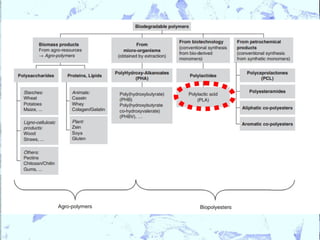
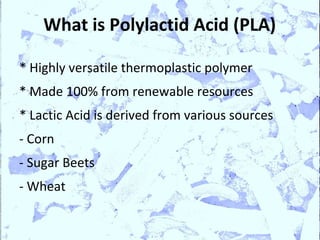
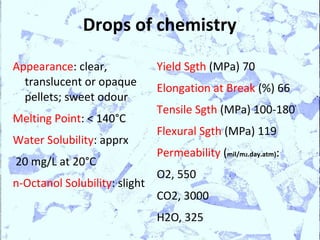
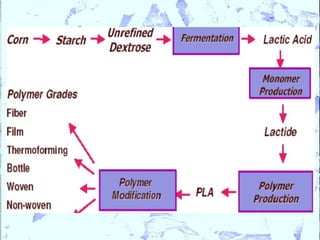
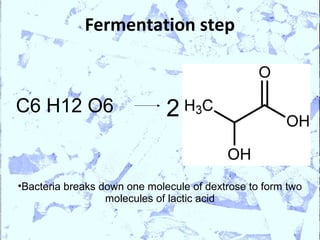
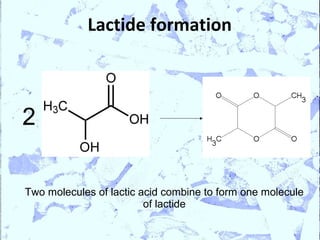
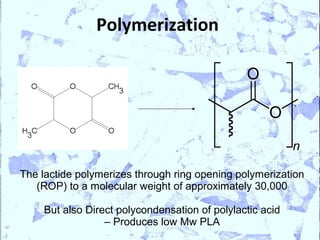
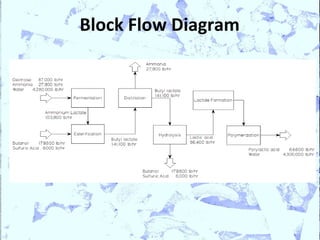
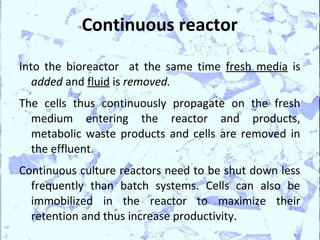
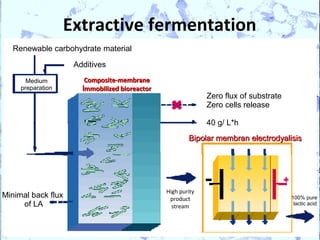
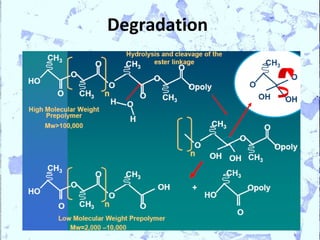
Poly Lactic Acid (PLA) is a biodegradable and compostable thermoplastic polymer made from renewable resources like corn, sugar beets and wheat. PLA is produced through fermentation of carbohydrates to lactic acid, then polymerization to form polylactic acid. It has physical properties comparable to polyethylene terephthalate but requires less fossil fuels to produce. While PLA has potential applications for single-use items and packaging due to its sustainability, its production also has criticisms related to energy usage and slowed degradation with certain additives.