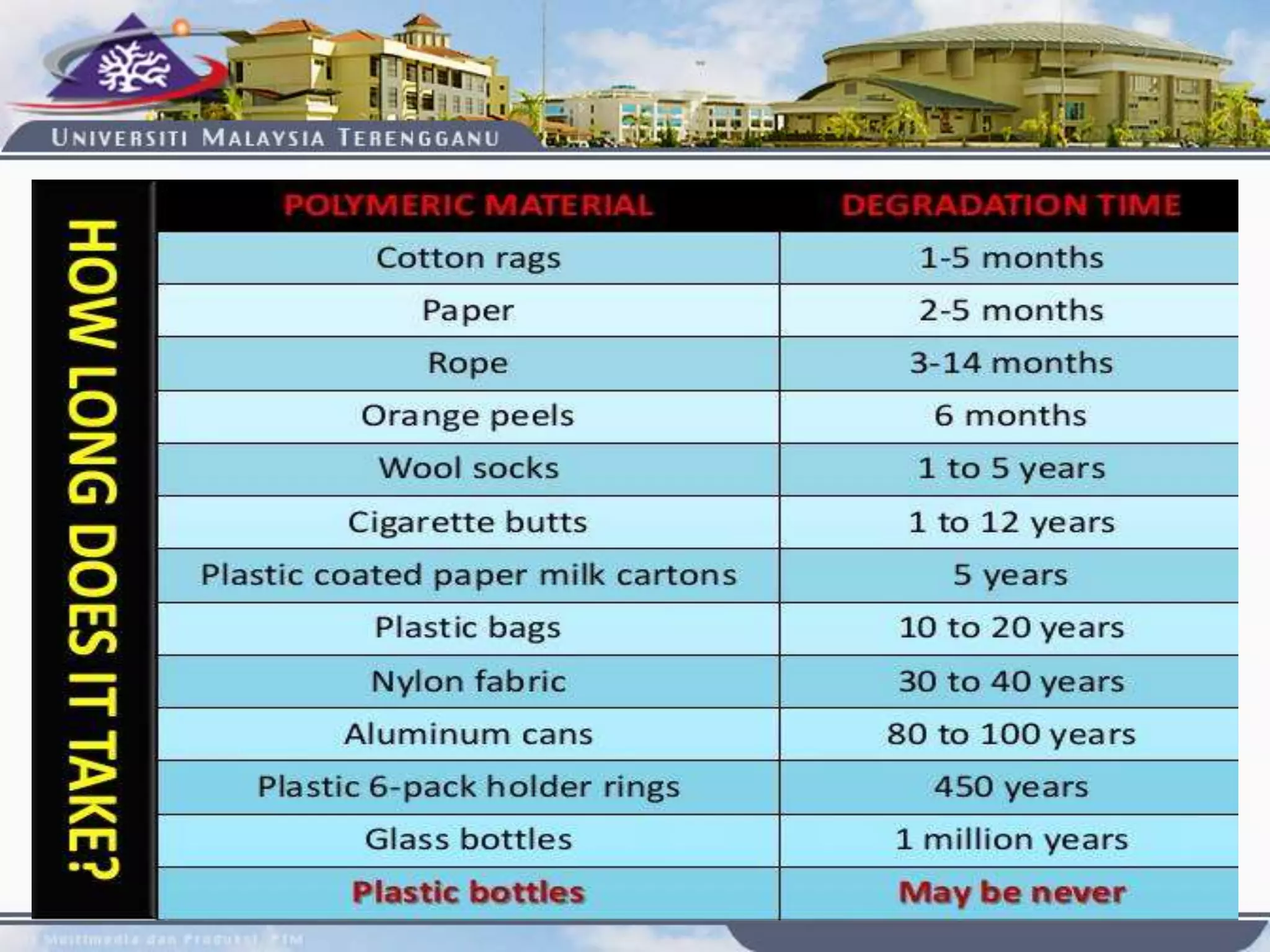
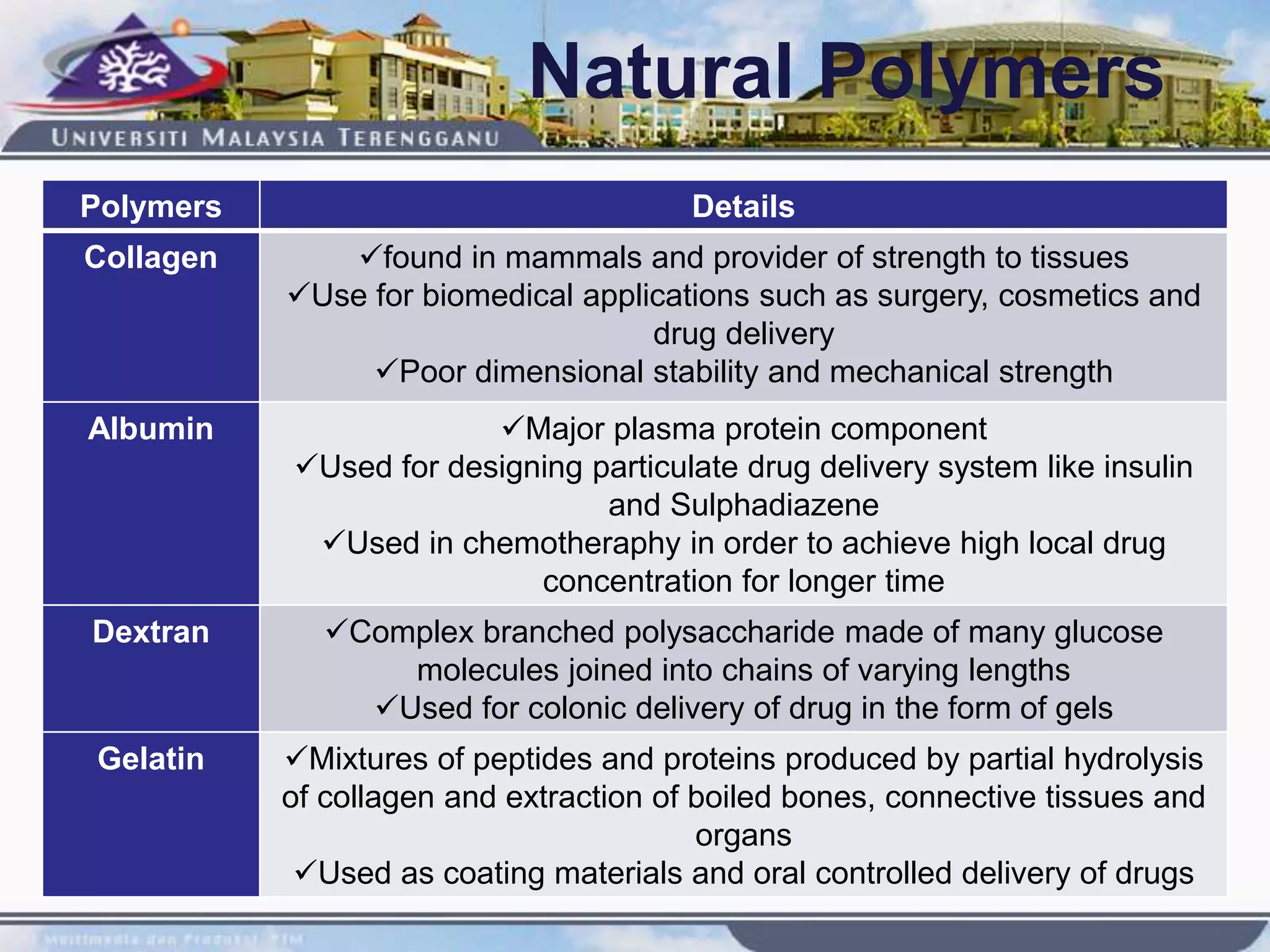
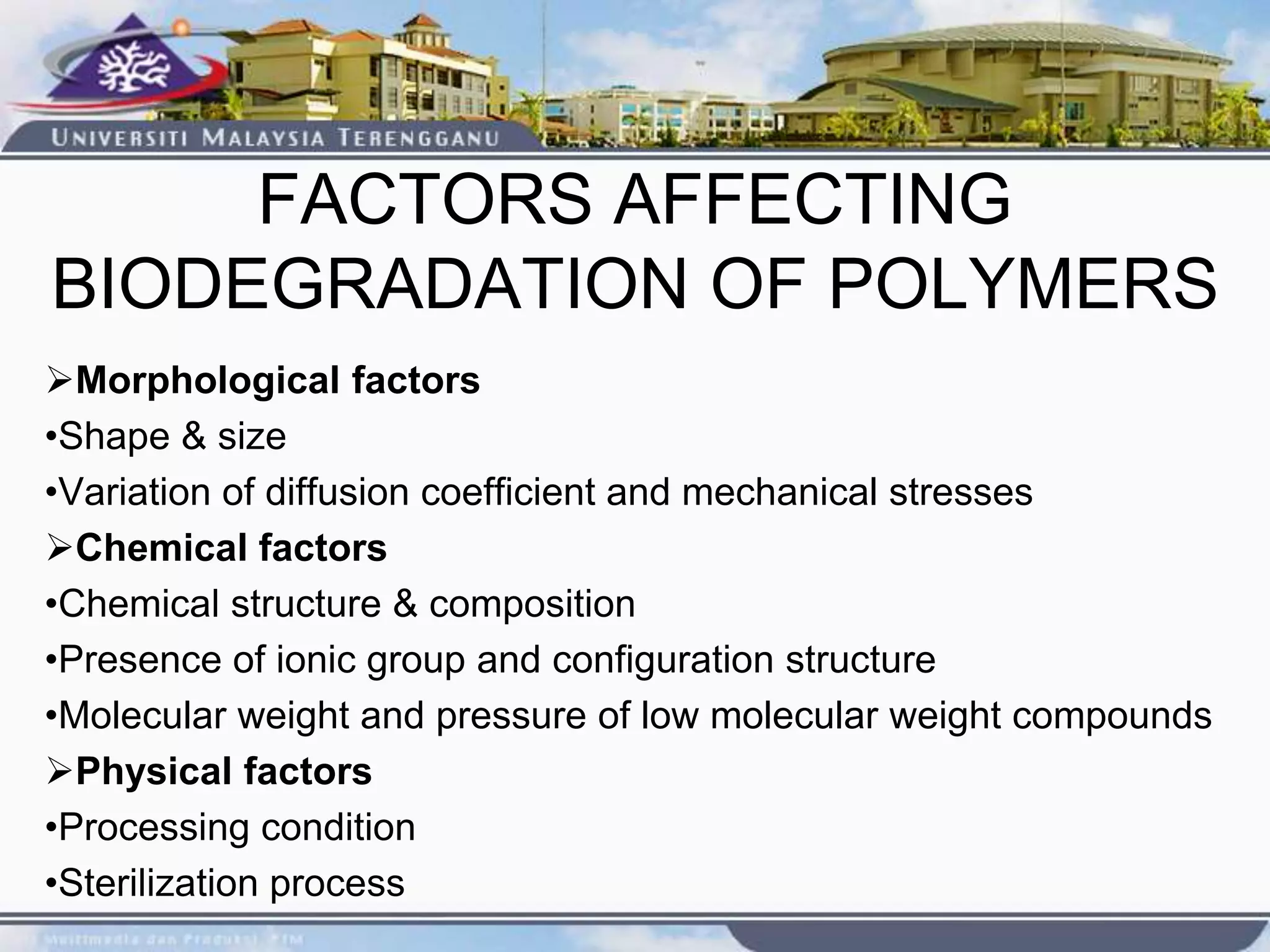
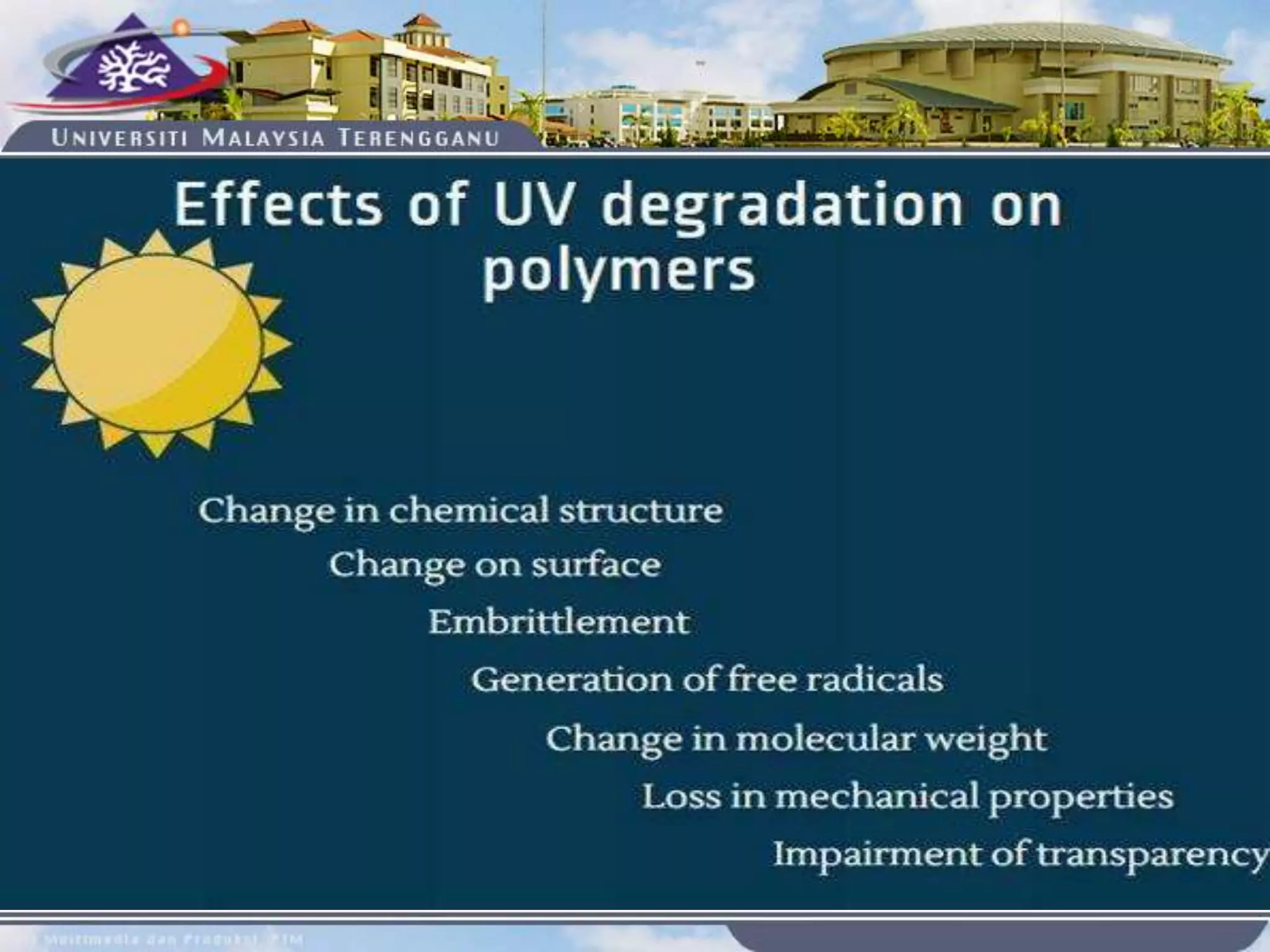
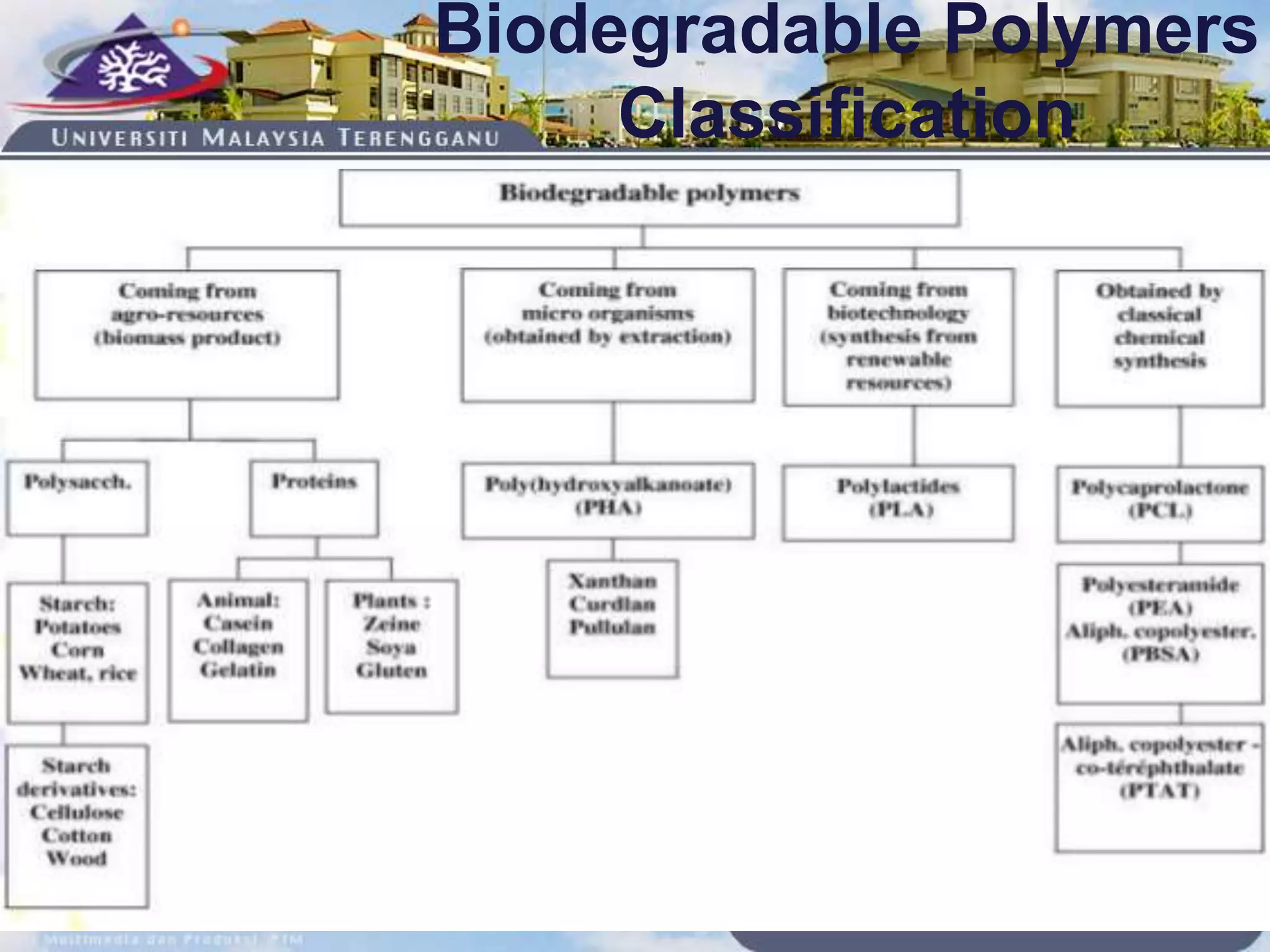
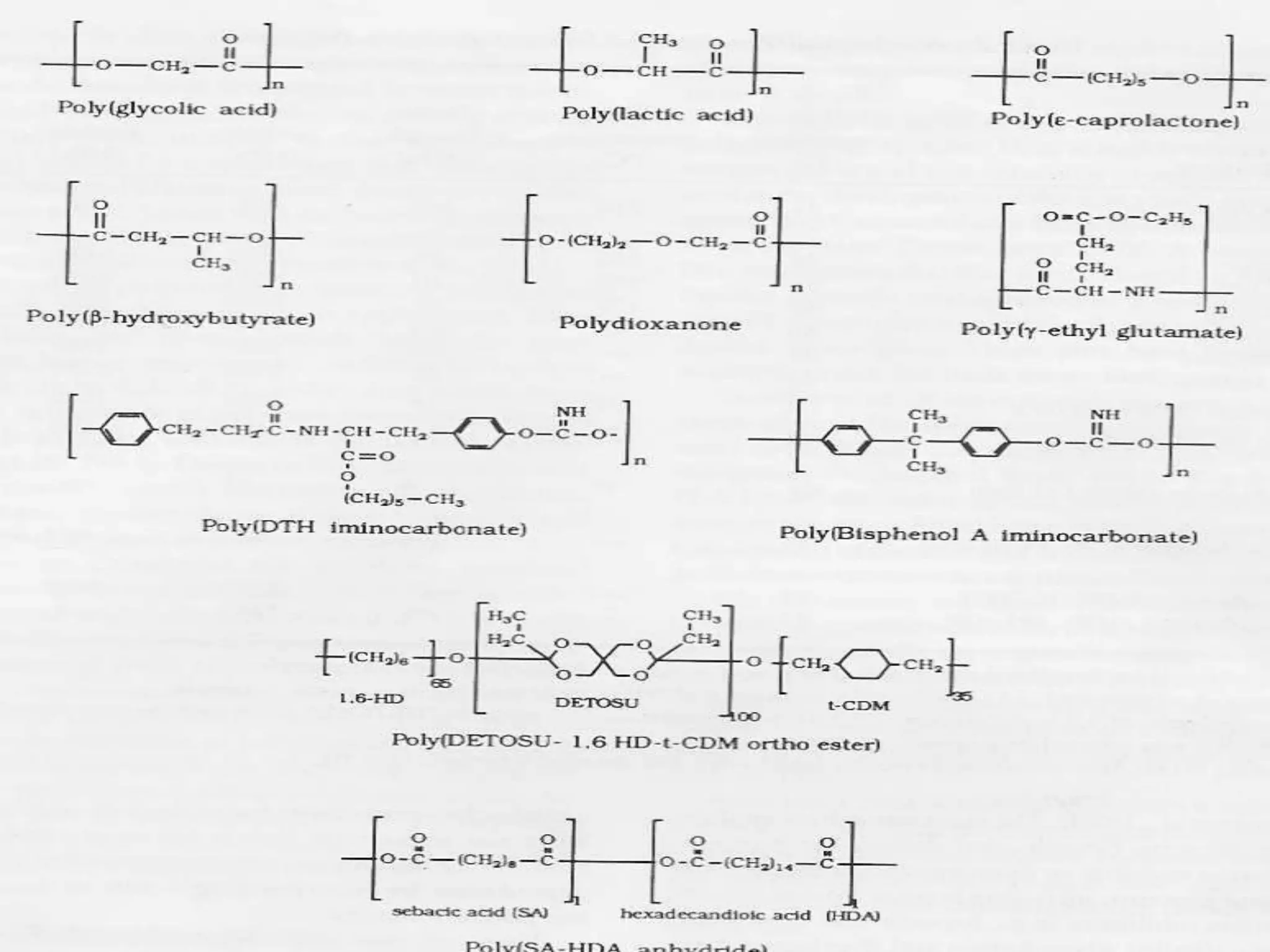
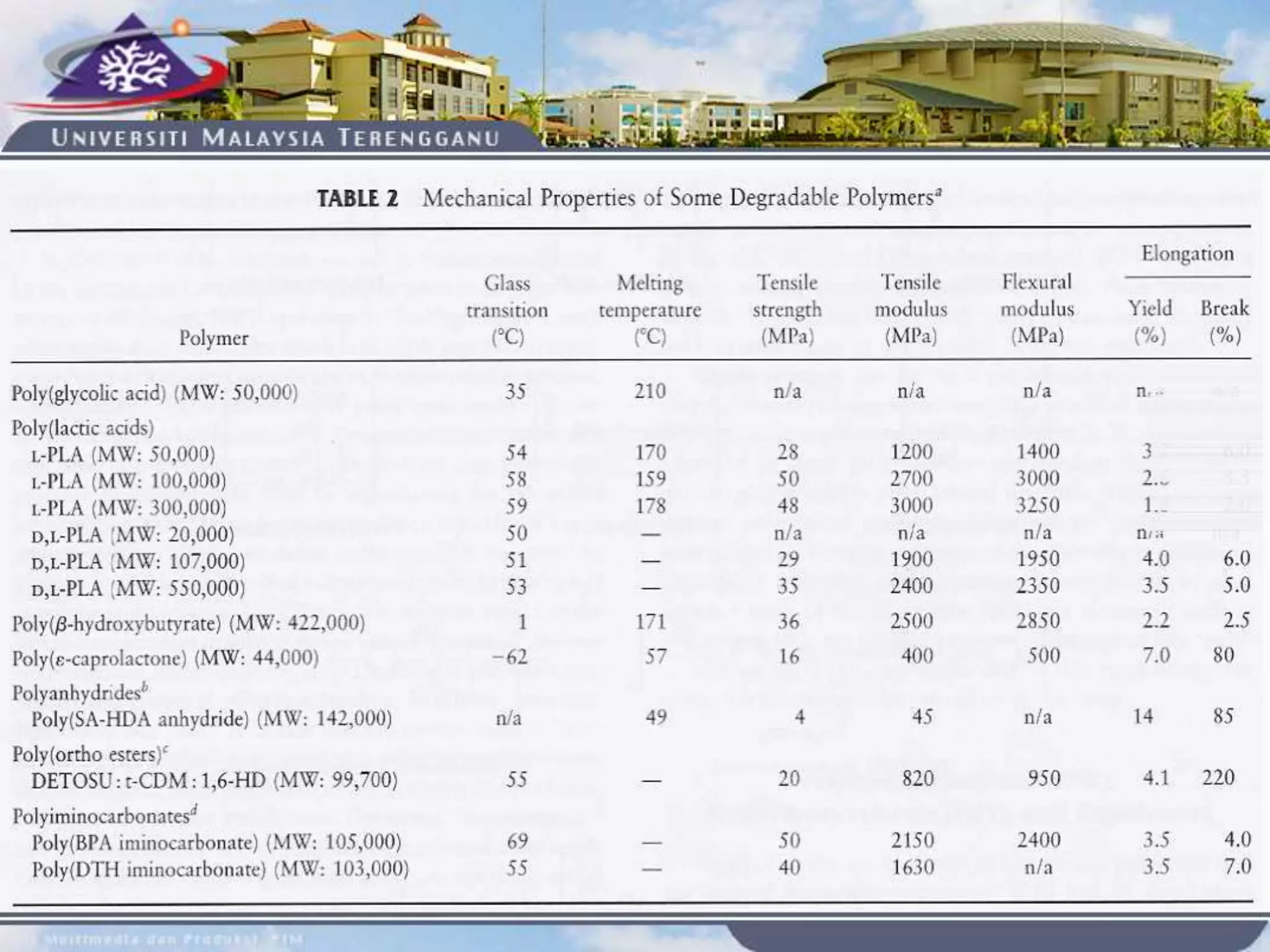
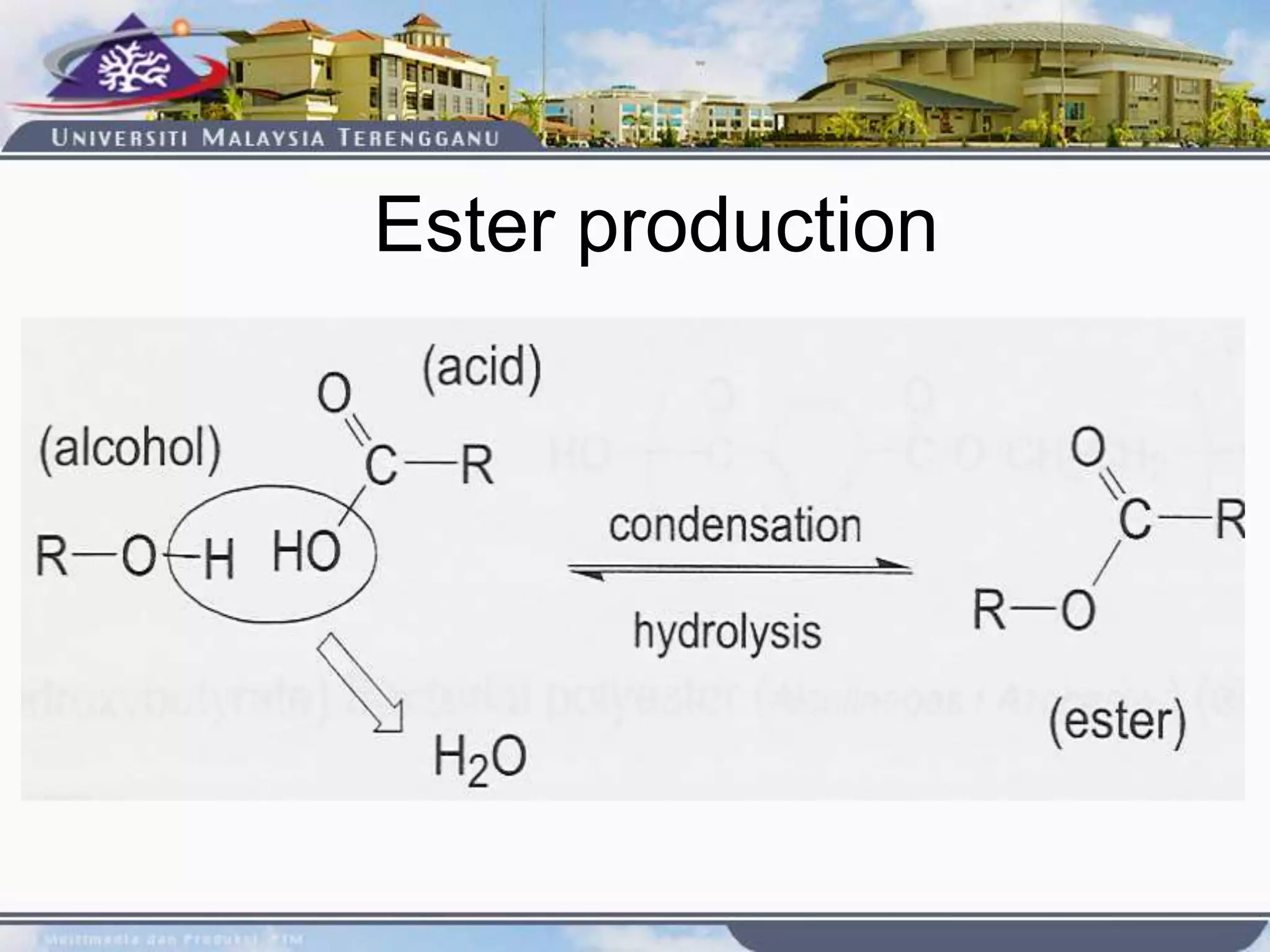
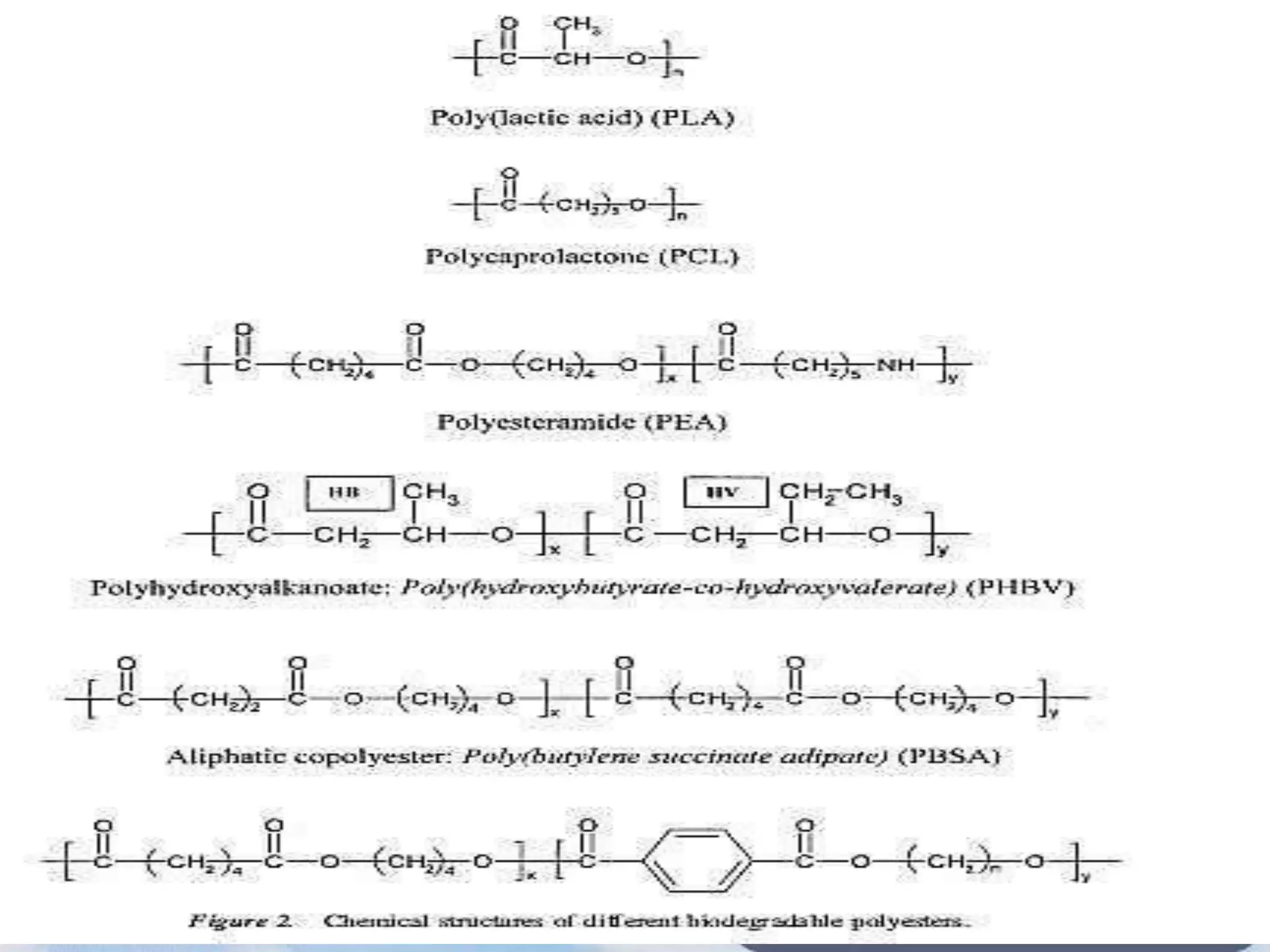
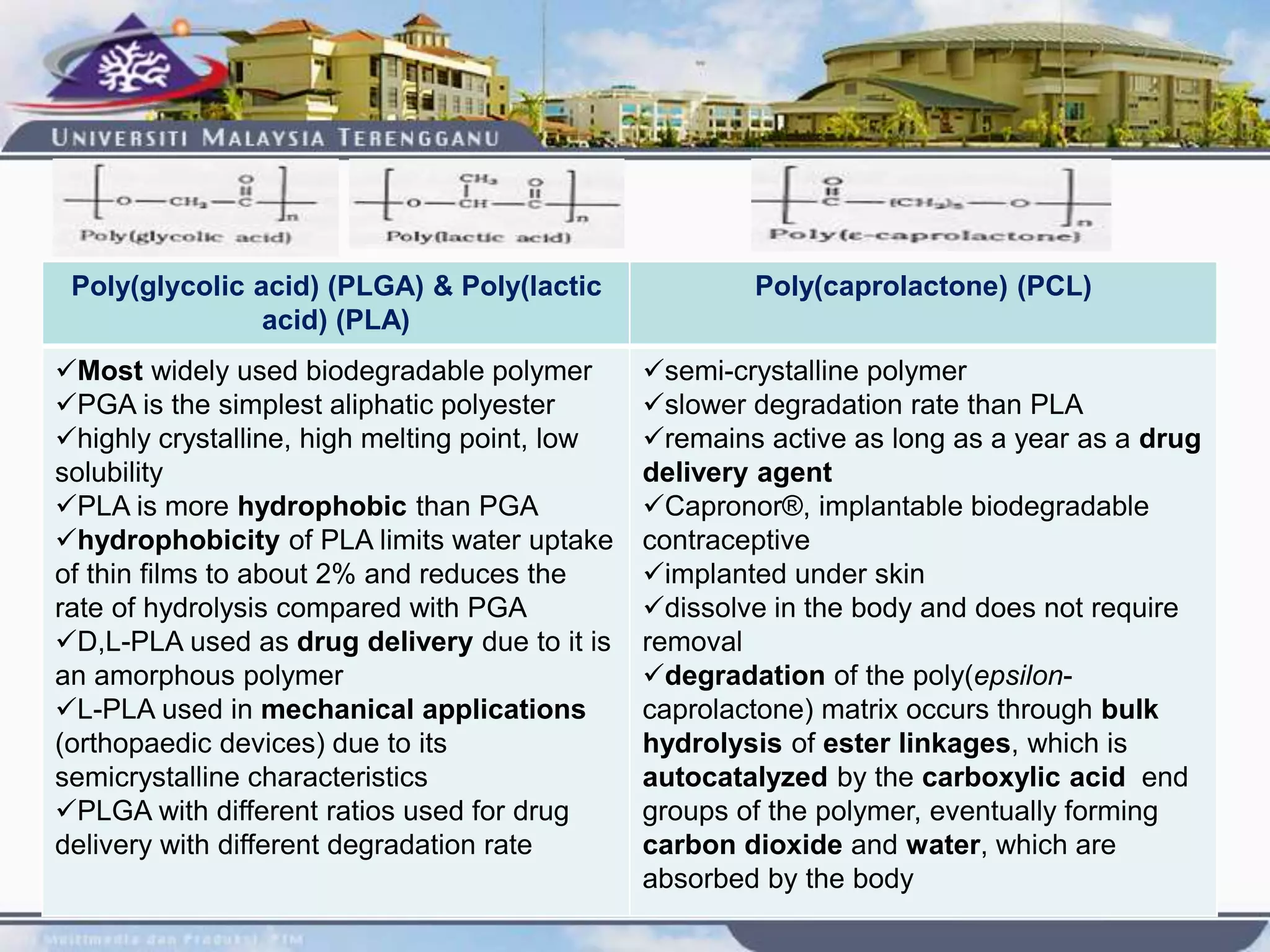
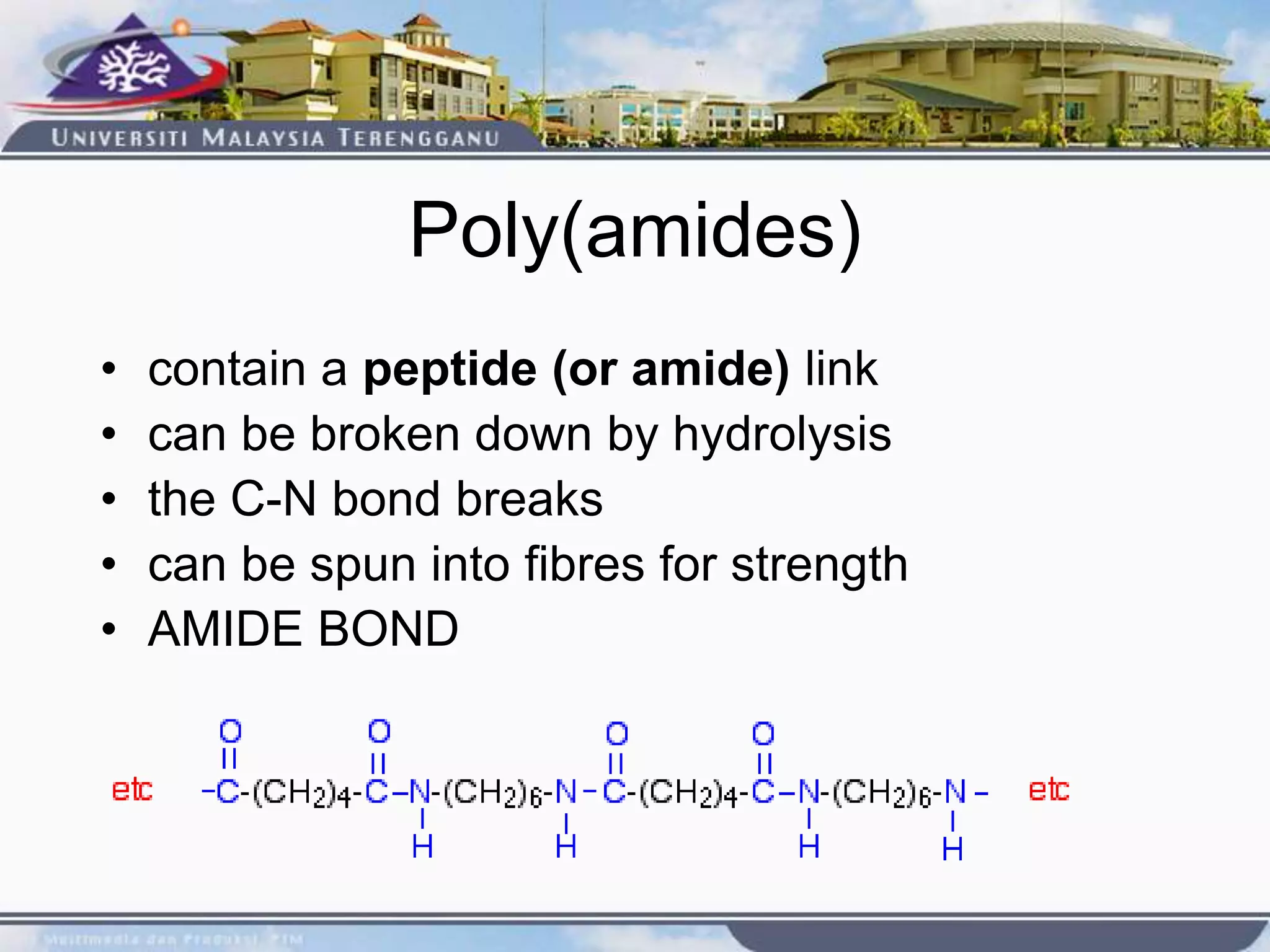
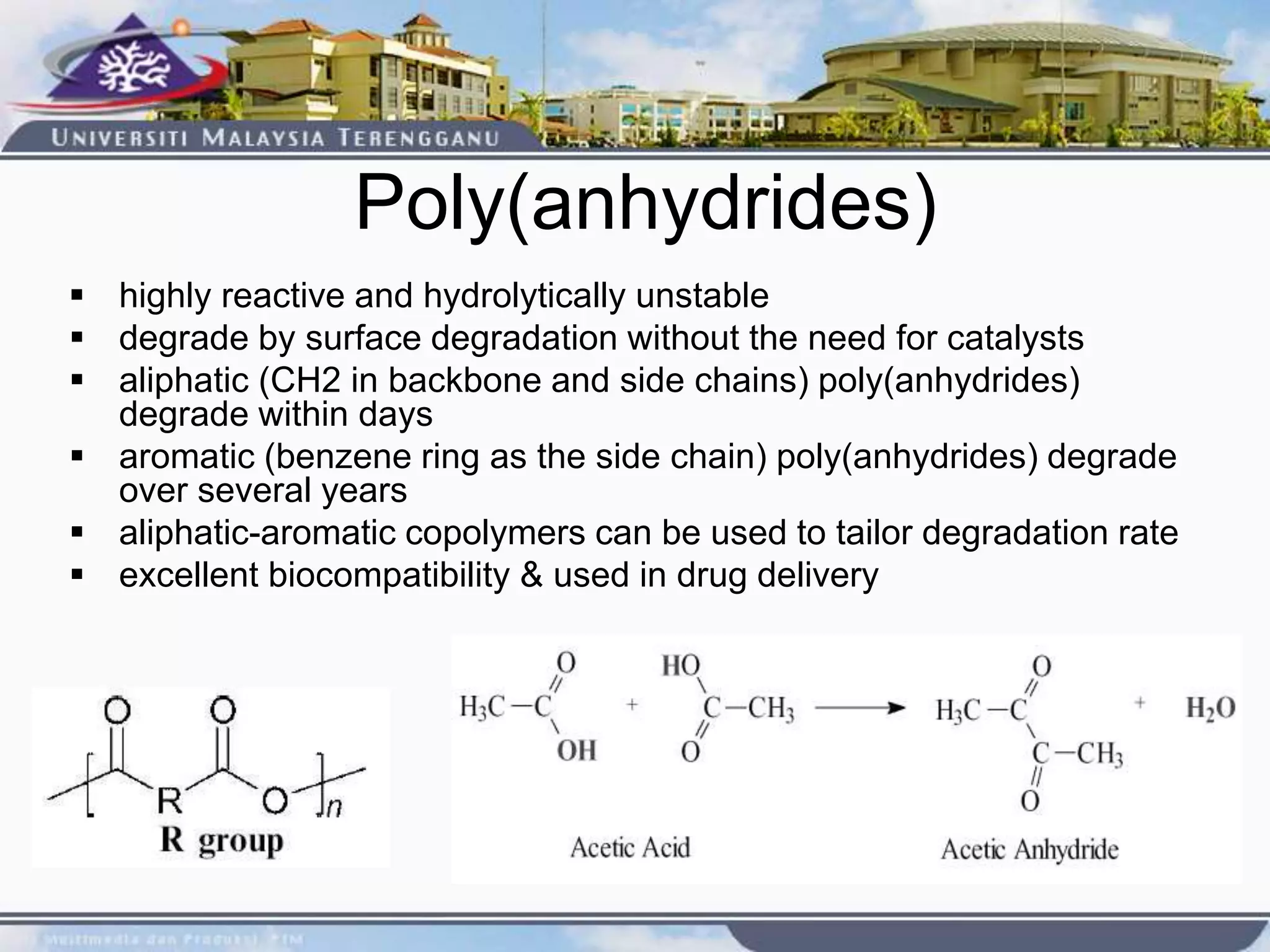
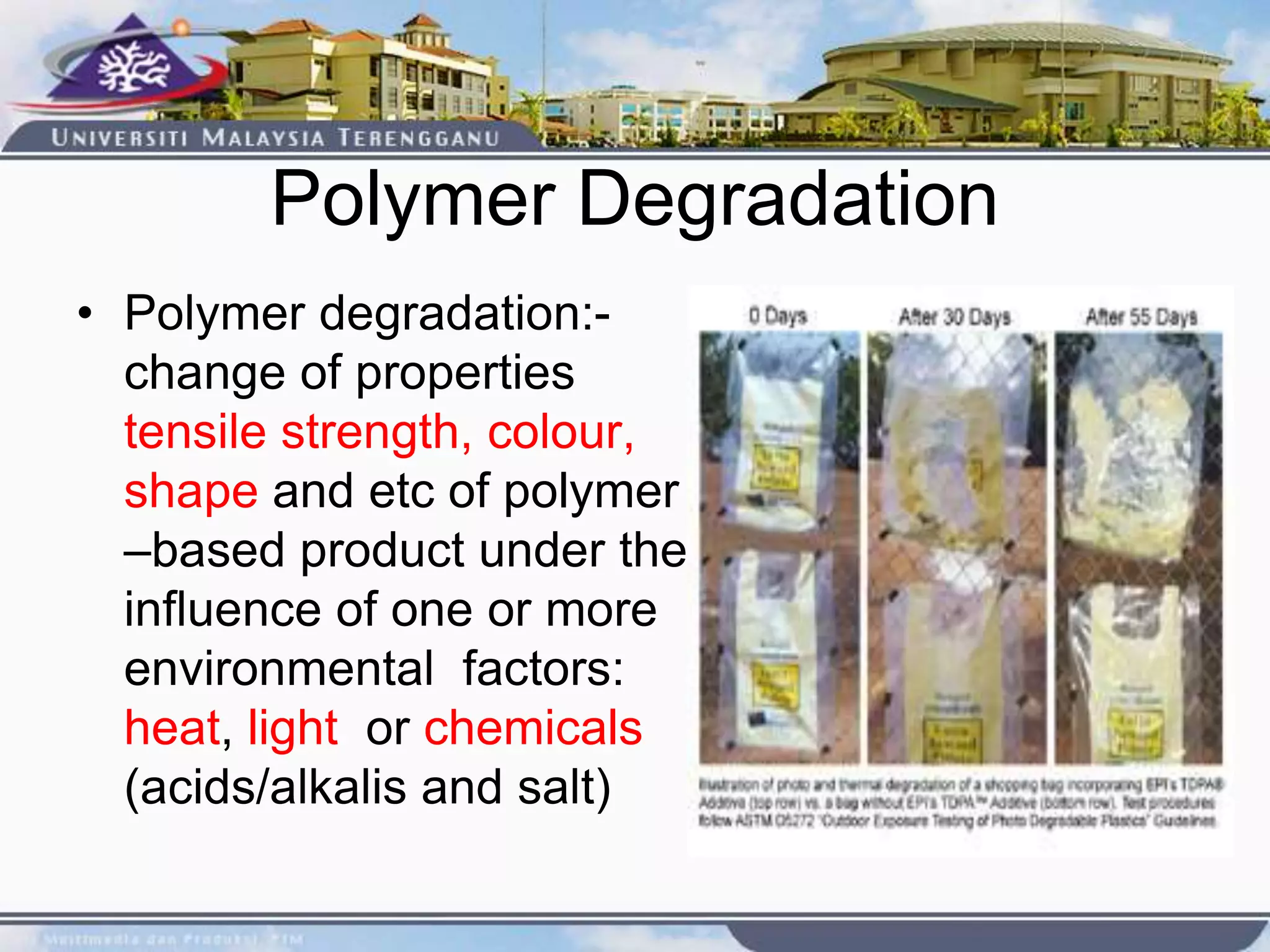
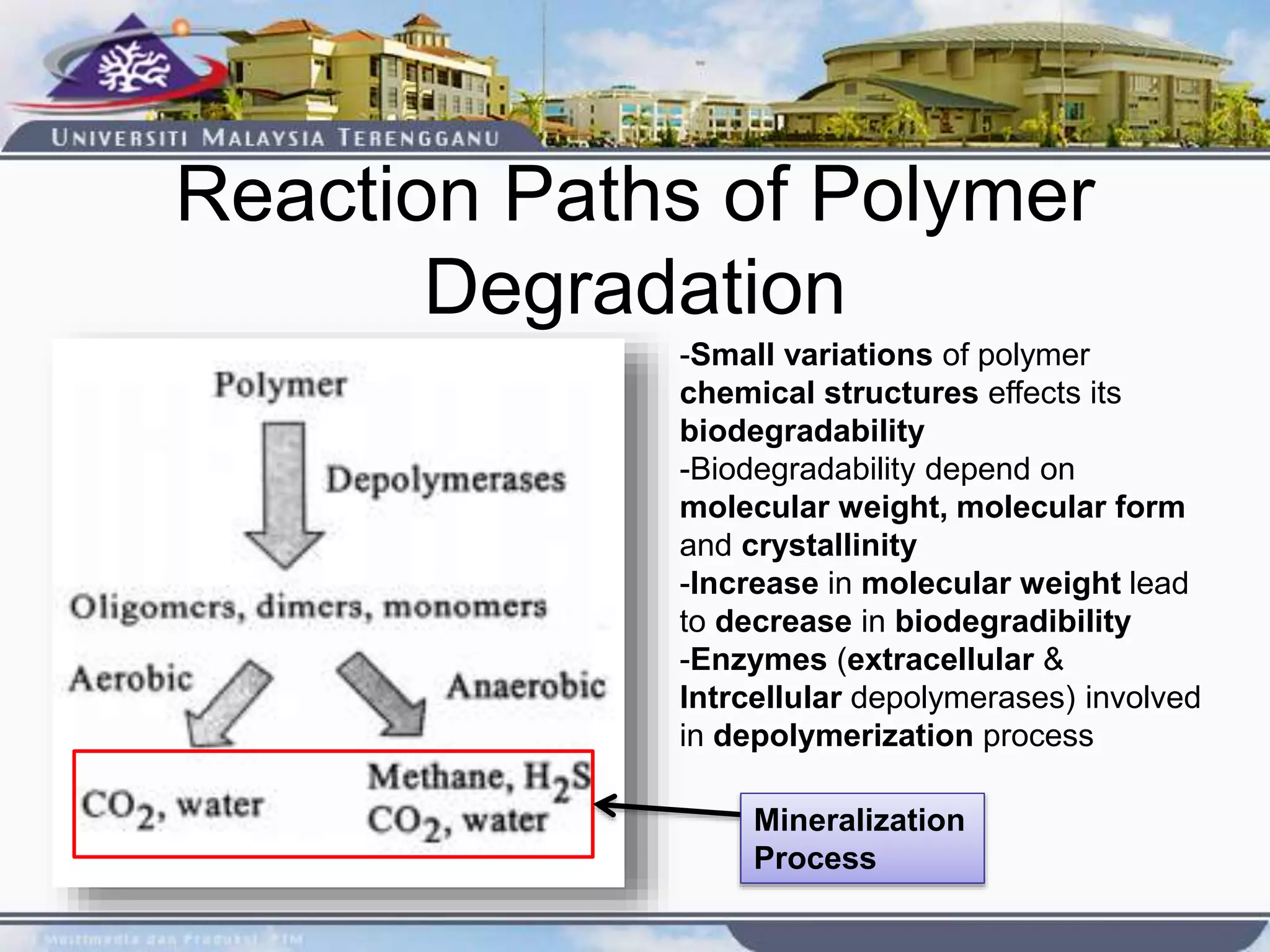
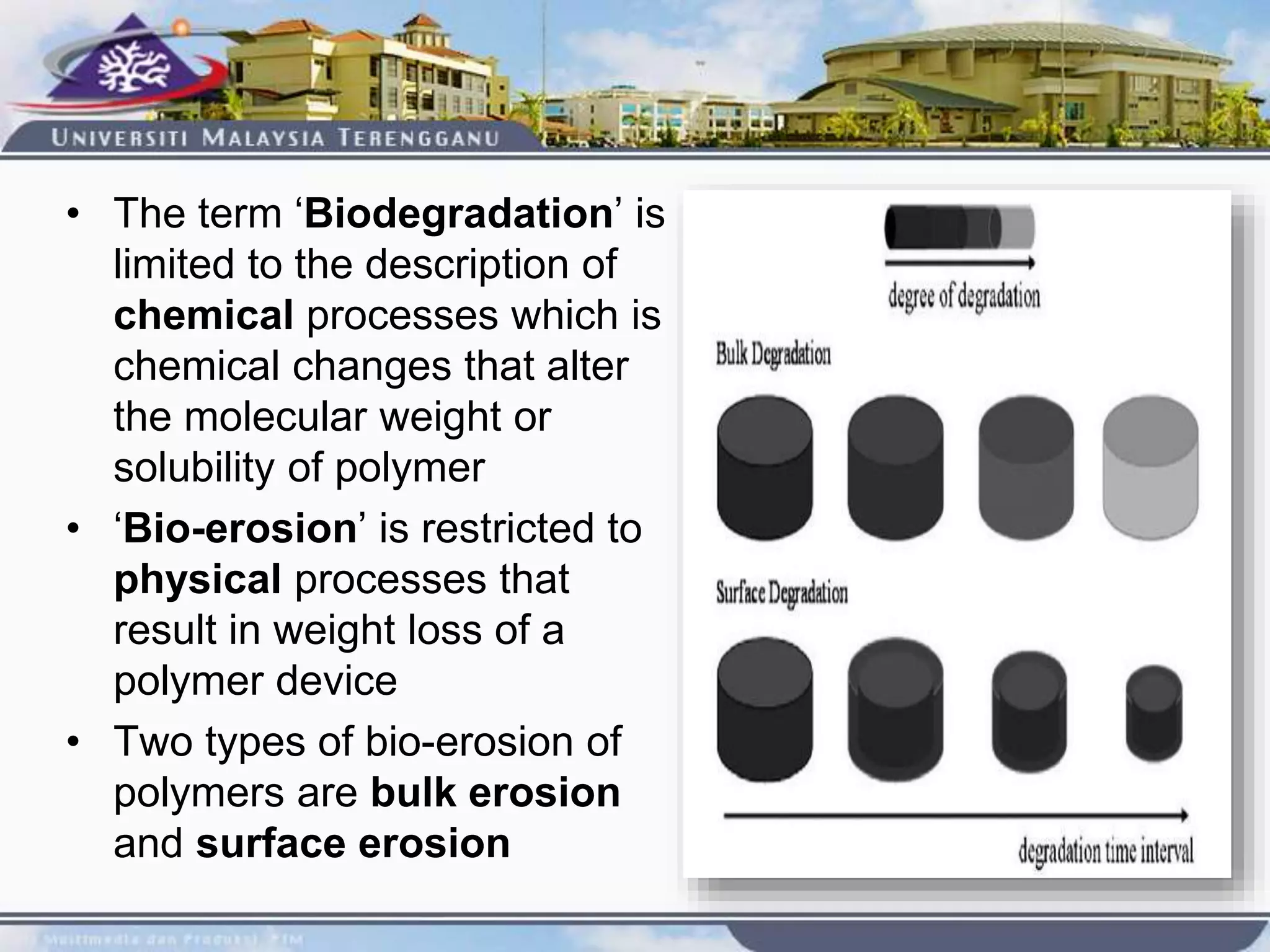
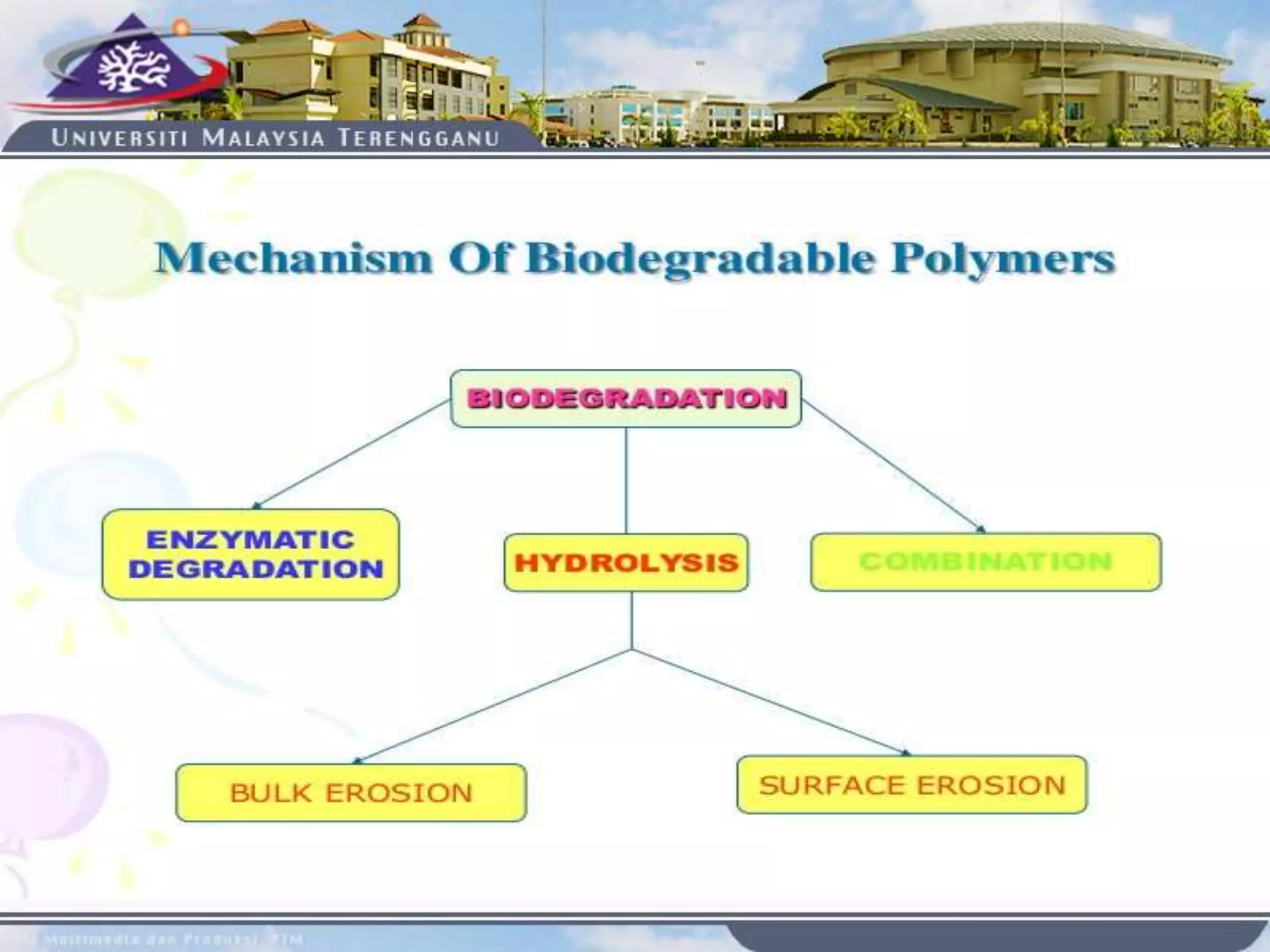
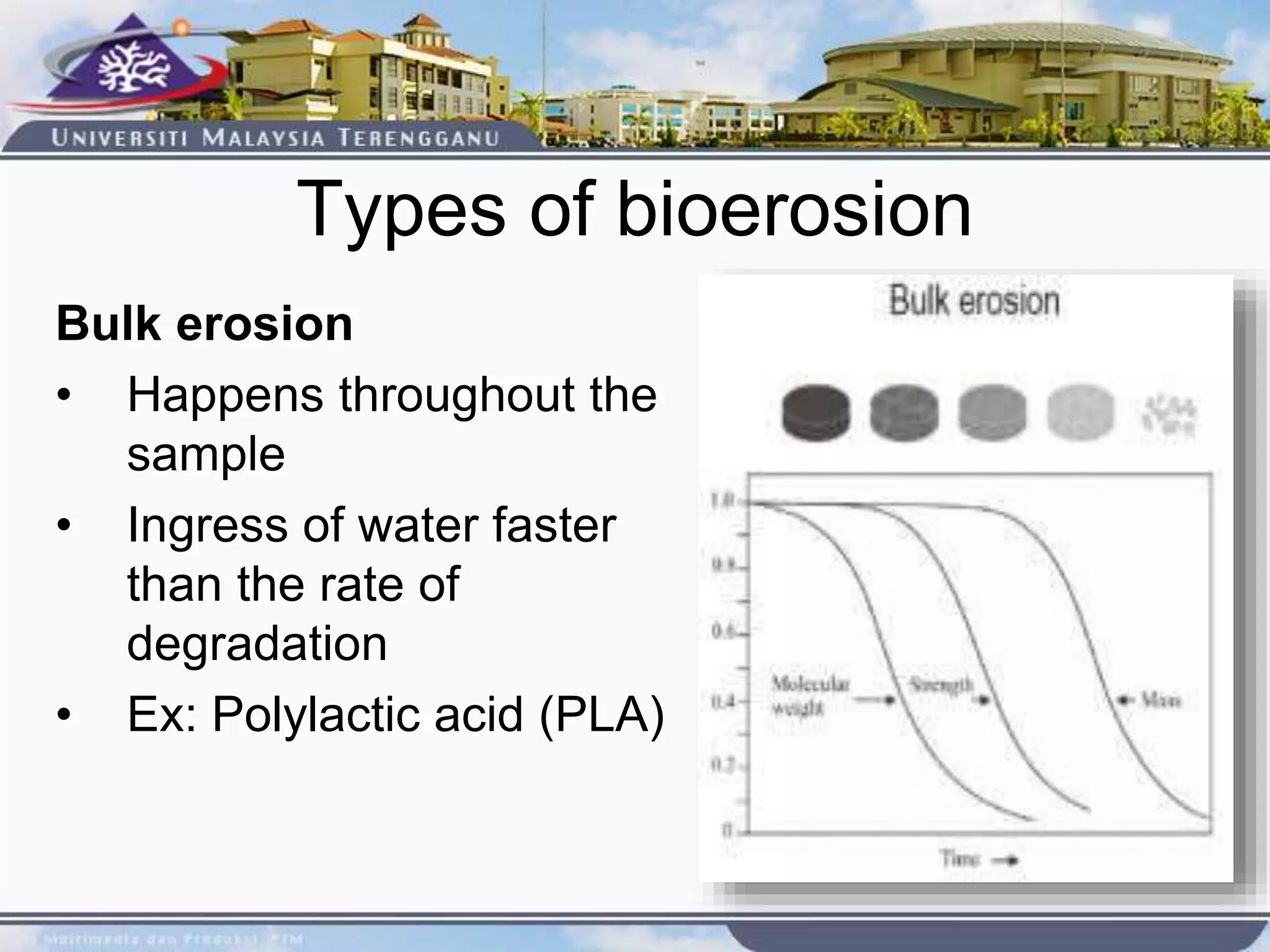
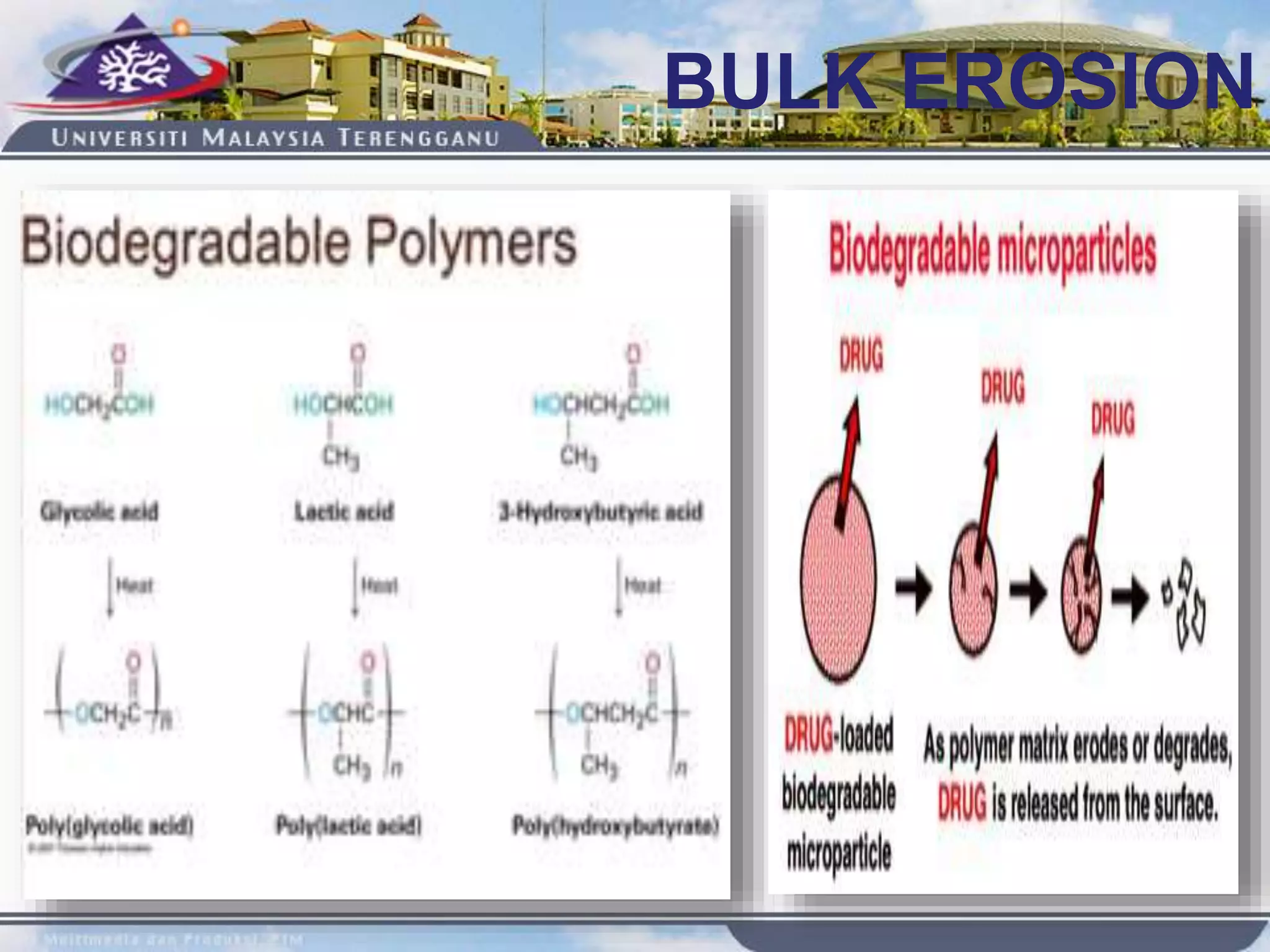
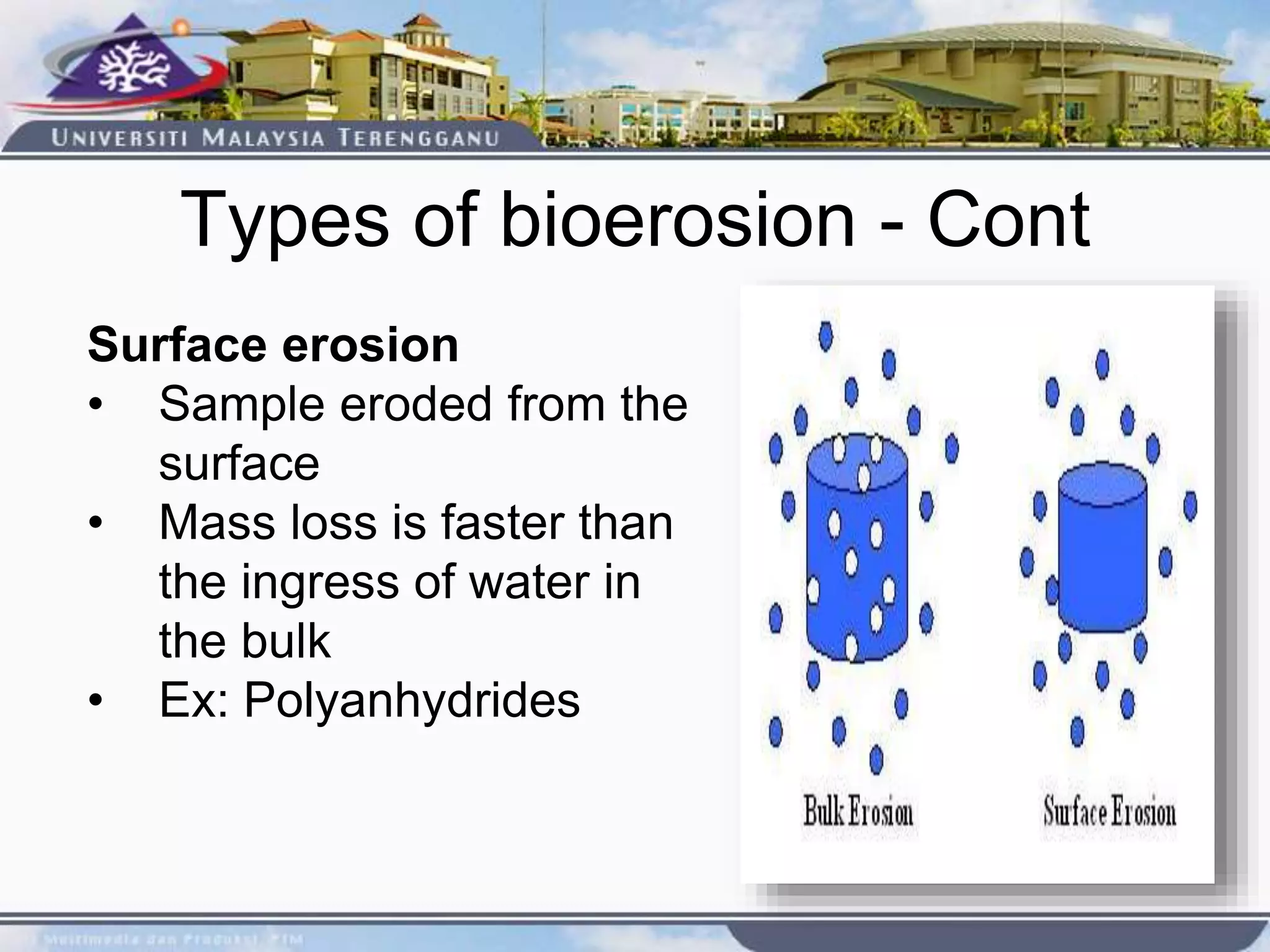
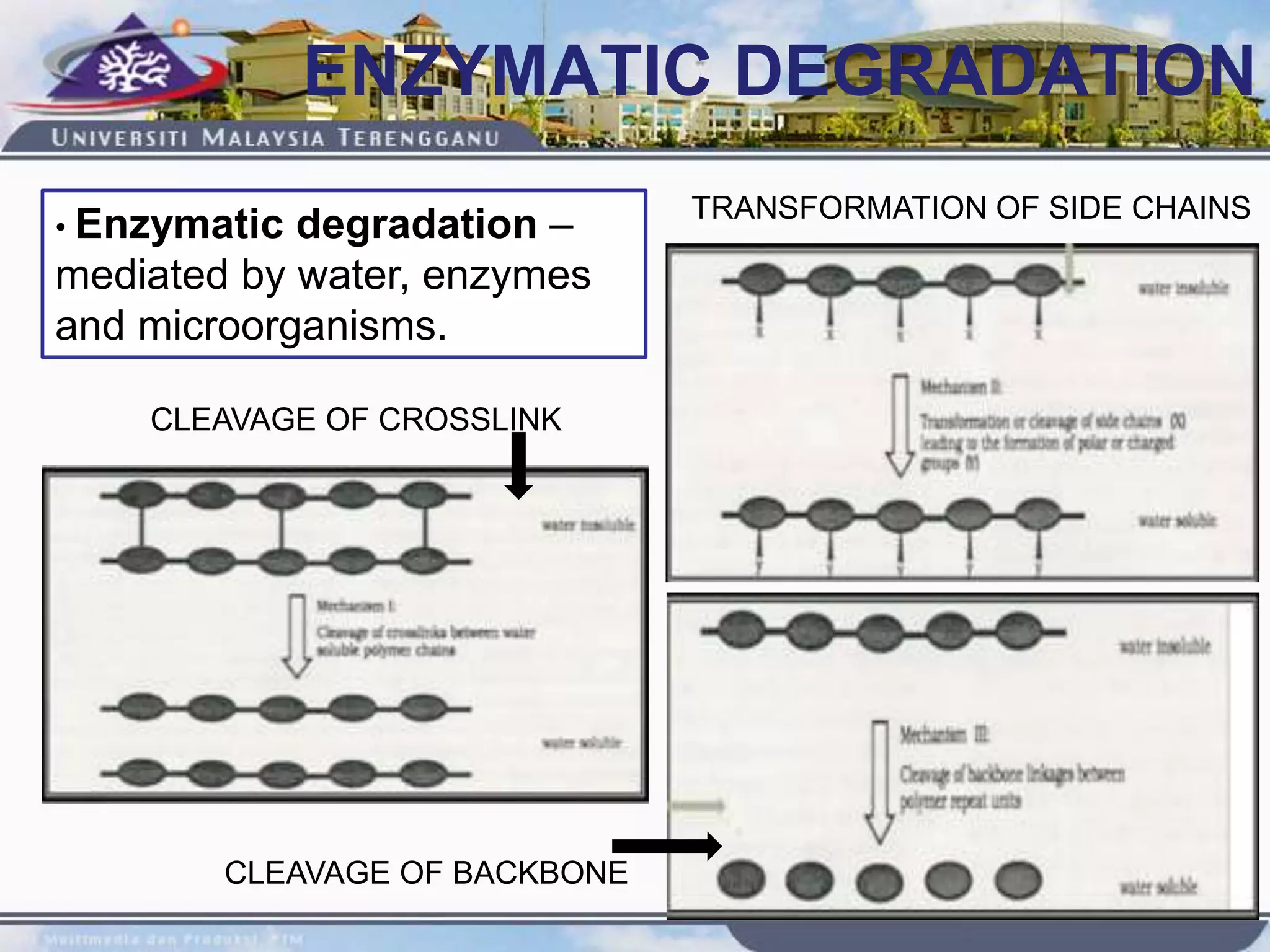
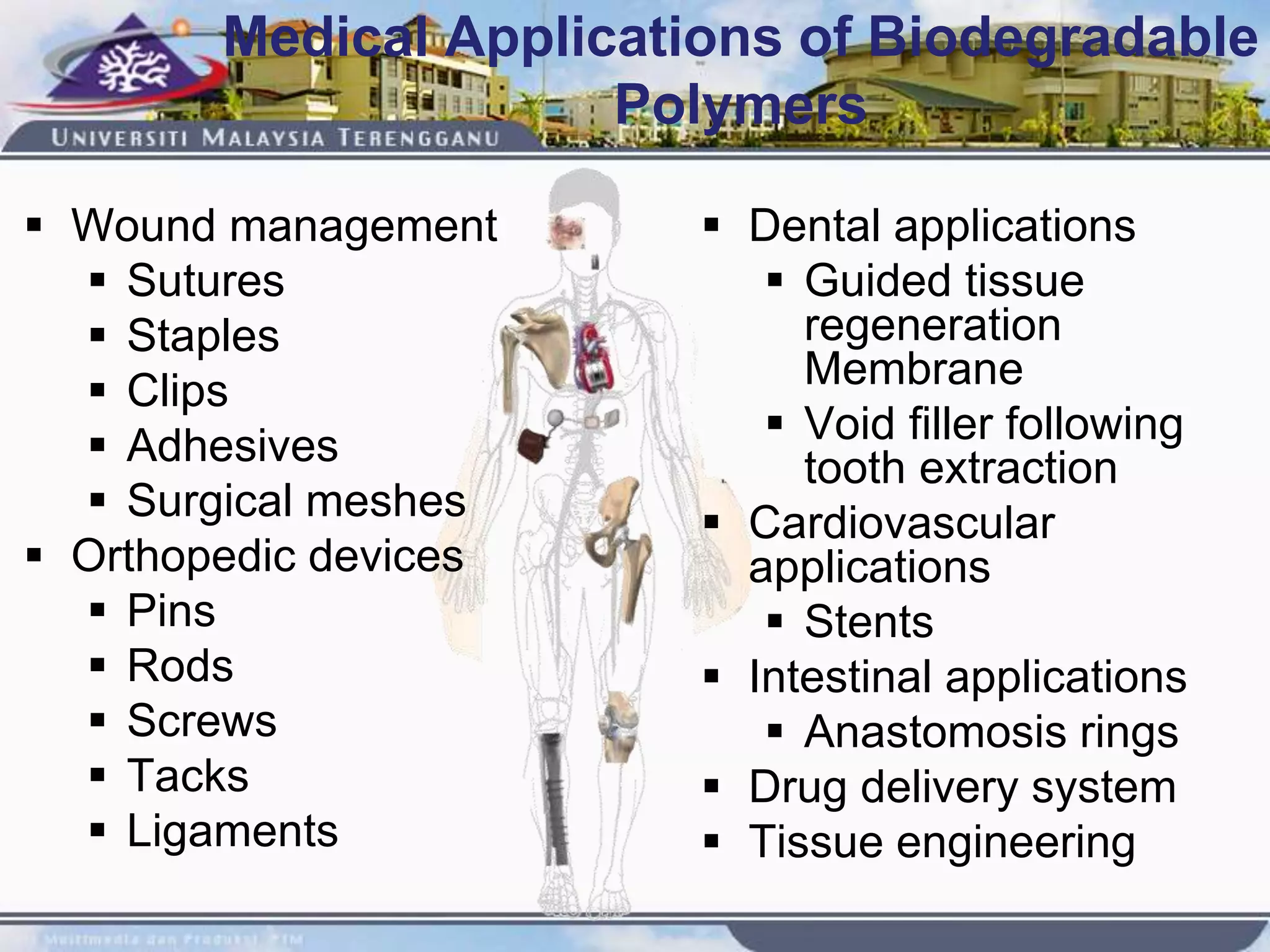
This document provides an overview of biodegradation of polymers. It begins with definitions of key terms like biodegradable polymer and discusses various factors that affect biodegradation like chemical structure, morphology, and physical properties. It then classifies polymers as natural or synthetic and lists examples of commonly used biodegradable polymers like poly(lactic acid), poly(glycolic acid), and poly(caprolactone). The mechanisms of biodegradation and bioerosion are described. Applications of biodegradable polymers in medical devices and advantages of using biodegradable polymers are highlighted. The document concludes with a glossary of terms.