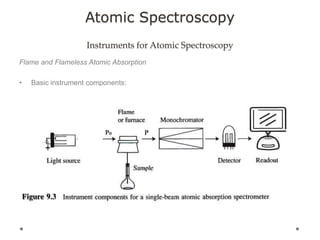
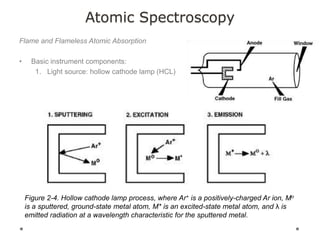
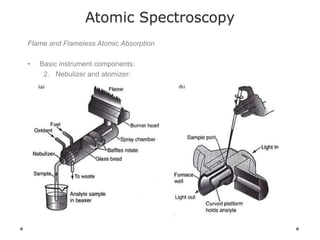
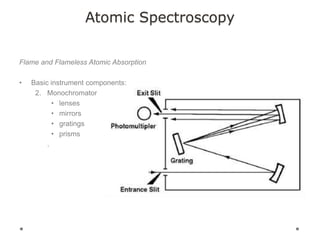
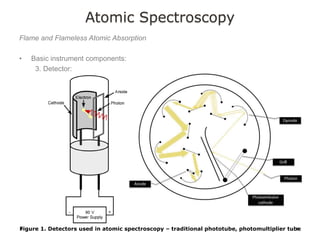
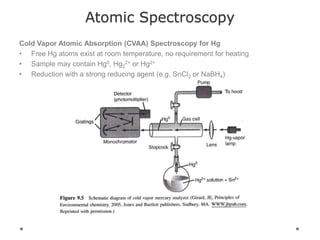
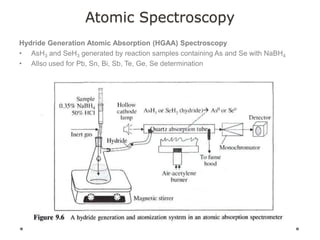
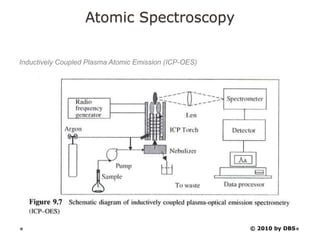
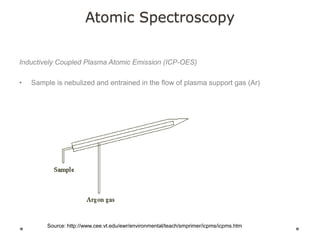
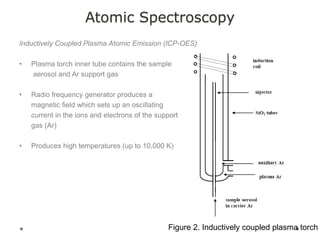
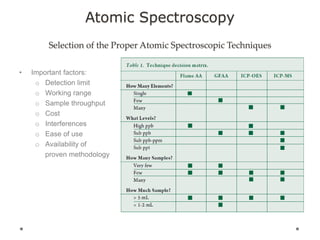
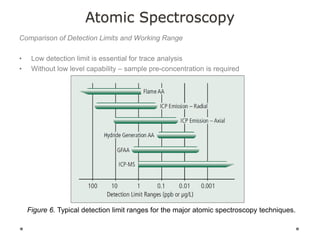
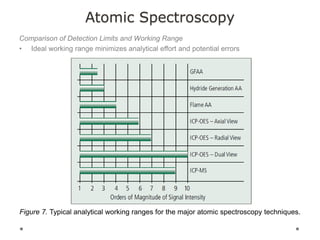
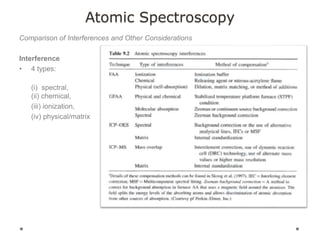
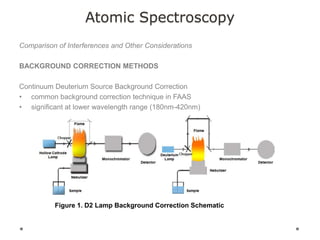
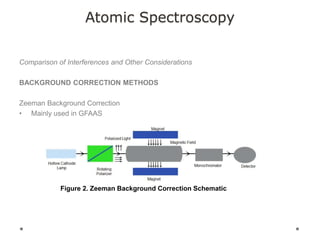
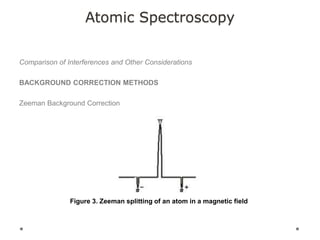
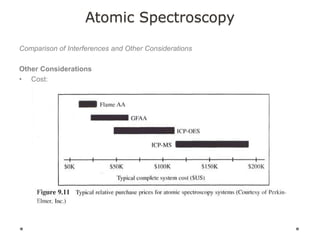
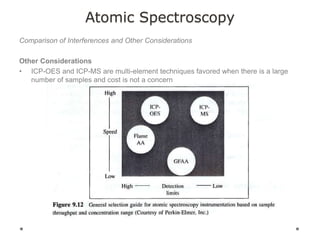
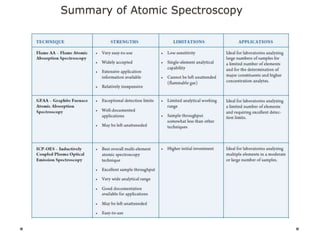
This document discusses principles and techniques in atomic absorption/emission spectroscopy. It describes the basic components and workings of flame atomic absorption, graphite furnace atomic absorption, inductively coupled plasma atomic emission spectroscopy, and their applications in elemental analysis. Factors for selecting the proper atomic spectroscopy technique include detection limits, working range, sample throughput, cost, interferences, ease of use, and availability of proven methodology. ICP-OES has become dominant for routine multi-element analysis due to its lower interferences, ability to analyze multiple elements simultaneously, and capacity to analyze non-metals.