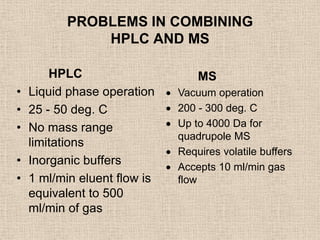
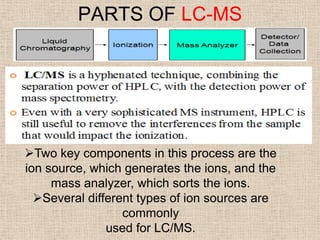
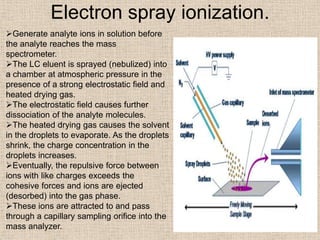
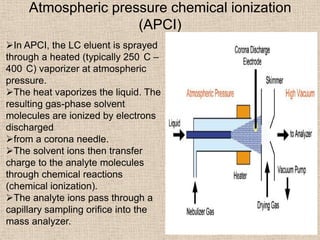
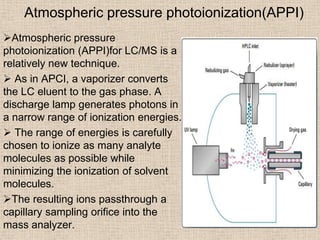
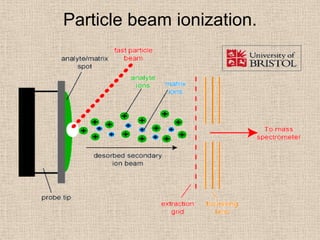
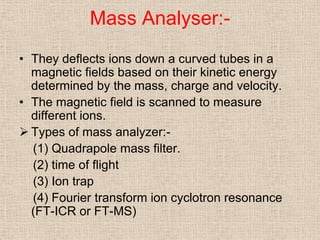
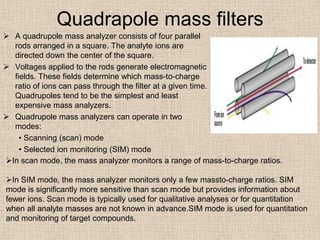
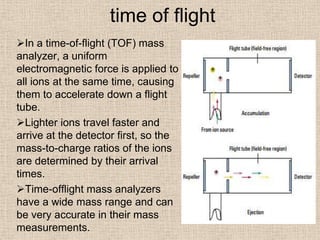
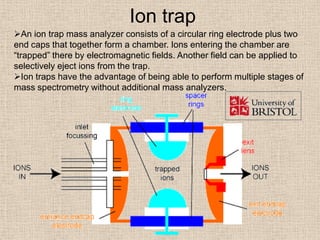
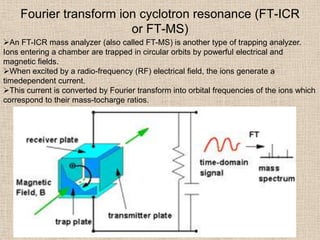
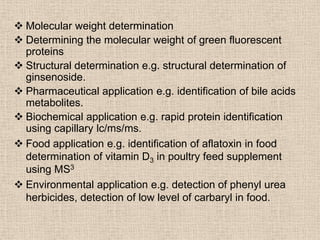
LC-MS combines liquid chromatography with mass spectrometry. It involves removing the detector from the LC column and interfacing the column directly with the mass spectrometer. The two key components are the ion source, which generates ions, and the mass analyzer, which sorts the ions. Common ion sources used include electrospray ionization, atmospheric pressure chemical ionization, and atmospheric pressure photoionization. Popular mass analyzers are quadrupole, time-of-flight, ion trap, and Fourier transform ion cyclotron resonance. LC-MS has applications in fields like molecular weight determination, structural determination, pharmaceutical analysis, food safety testing, and environmental analysis.