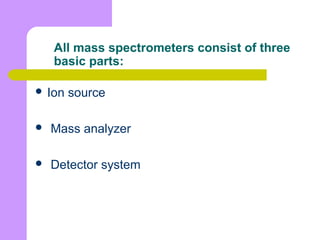
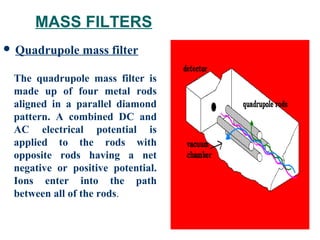
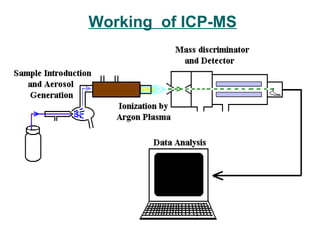
This document provides an overview of inductively coupled plasma mass spectrometry (ICP-MS). ICP-MS uses a plasma to atomize and ionize elemental samples and then a mass spectrometer to separate and detect ions to determine elemental composition. Key components include the sample introduction system, plasma torch, mass filter, and detector. ICP-MS can detect over 70 elements at very low concentrations (parts-per-trillion levels) and is widely used in applications like semiconductor analysis, biological research, and geochemistry. Limitations include potential matrix effects and polyatomic interferences.