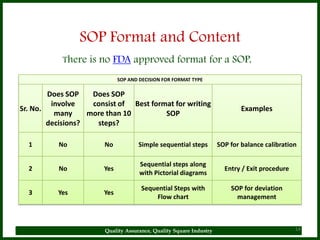
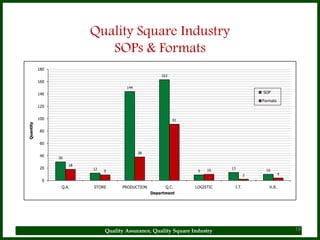
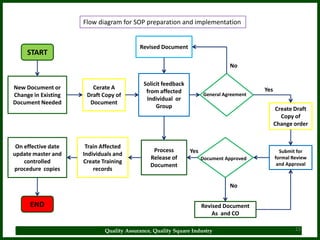
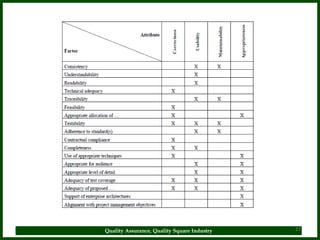
The document provides a comprehensive overview of quality assurance and standard operating procedures (SOPs) in the pharmaceutical industry, emphasizing their importance for maintaining product quality and regulatory compliance. It outlines the purpose, preparation, formatting, and maintenance of SOPs, alongside their benefits, such as consistency in operations and compliance with manufacturing standards. Key points include the necessity for training on SOPs, the significance of document control, and the ongoing review process to ensure relevancy and effectiveness.





![Purpose Of SOP
[SCHEDULE M]
[See Rules 71, 74, 76 and 78]
• GOOD MANUFACTURING PRACTICES AND REQUIREMENTS OF
PREMISES, PLANT AND EQUIPMENT FOR PHARMACEUTICAL
PRODUCTS.
• Note: - To achieve the objectives listed below, each licensee shall evolve
appropriate methodology, systems and procedures which shall be
documented and maintained for inspection and reference; and the
manufacturing premises shall be used exclusively for production of drugs
and no other manufacturing activity shall be undertaken therein.
Quality Assurance, Quality Square Industry 6](https://image.slidesharecdn.com/sop-120924054559-phpapp02/85/SOP-6-320.jpg)