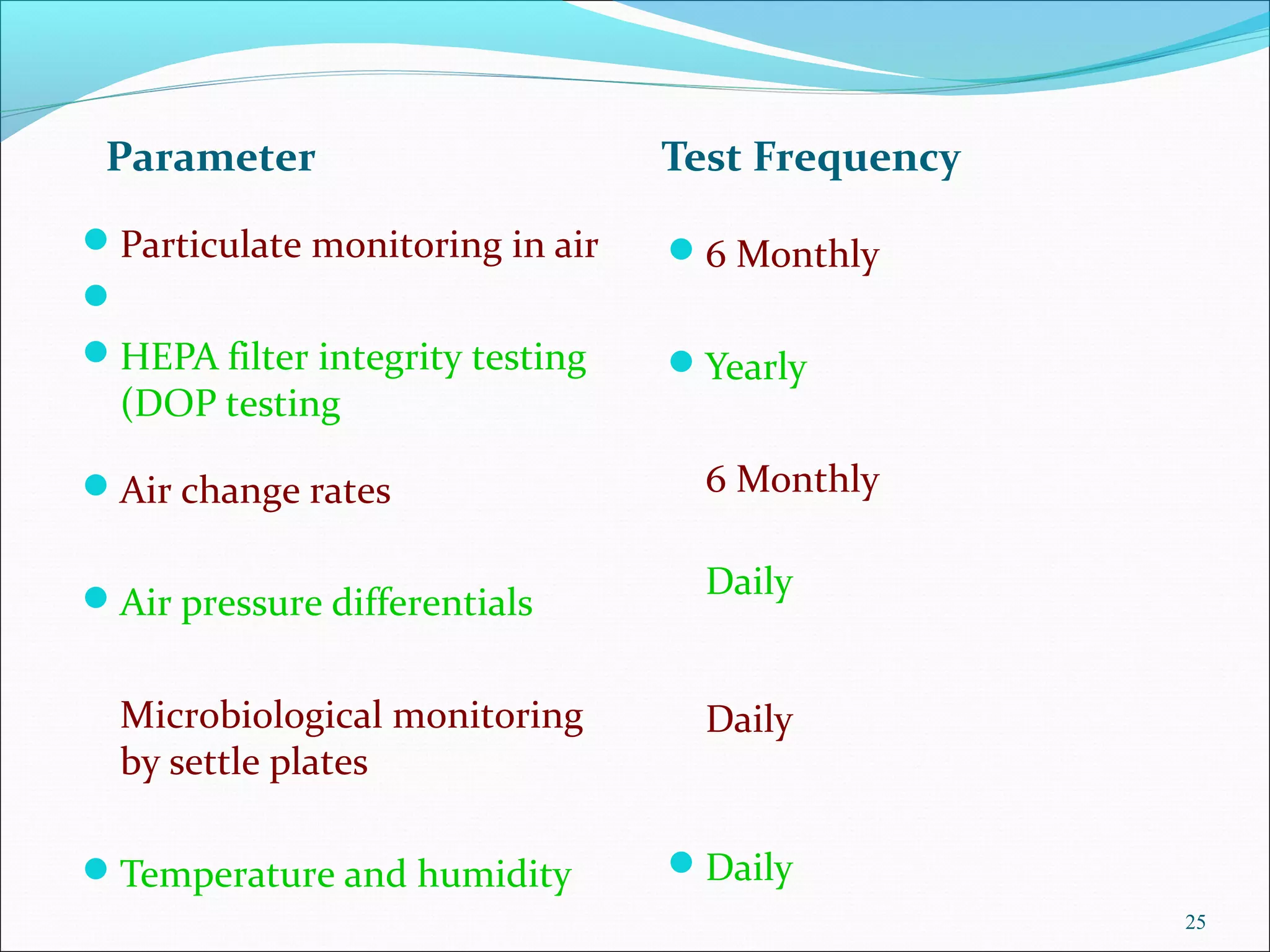
The document discusses the importance of environmental control in the pharmaceutical industry. It states that controlling factors like particulate matter, microorganisms, temperature, humidity and airflow is crucial to protecting pharmaceutical products from contamination. A properly designed, validated and monitored HVAC system is necessary to ensure quality and safety. The HVAC system controls air movement and distribution of temperature and humidity in cleanrooms to maintain suitable manufacturing conditions.