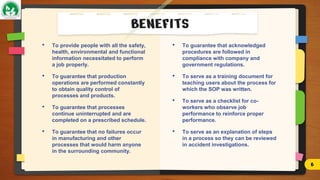
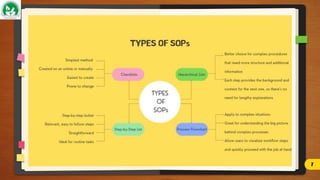
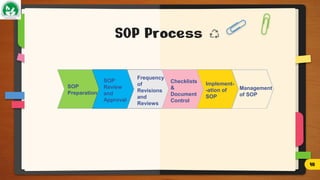
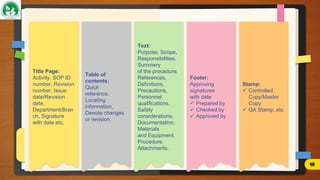
The document discusses Standard Operating Procedures (SOPs), which are detailed written instructions designed to ensure routine activities within organizations are performed consistently and efficiently, adhering to quality and regulatory standards. It outlines the objectives, benefits, types, writing styles, and processes related to SOPs, emphasizing the importance of training and management practices to maintain their effectiveness. Additionally, it provides guidance on SOP documentation and reviews, ensuring procedures remain current and applicable.