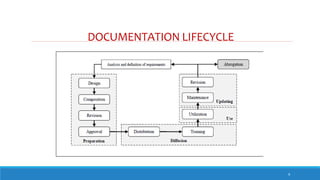
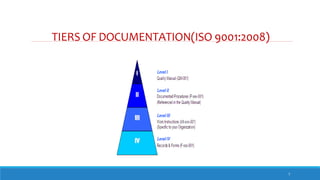
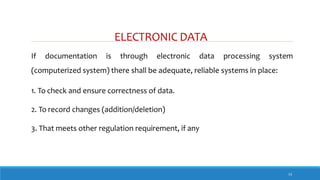
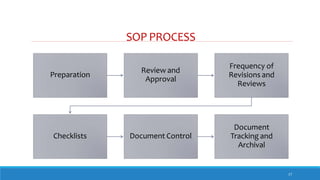
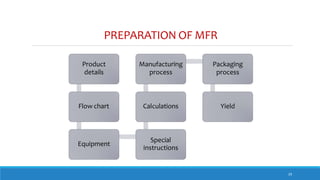
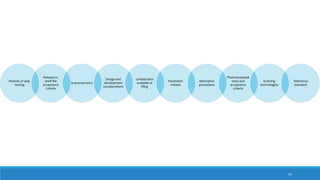
The document outlines the importance of documentation in pharmaceutical quality management systems, emphasizing the regulatory requirements and best practices necessary for compliance. It details the objectives, lifecycle, and characteristics of documentation, along with the need for standard operating procedures (SOPs) and audit processes to ensure quality control. Additionally, it highlights the significance of electronic data management in maintaining accurate records and compliance with good documentation practices.