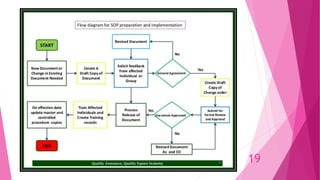
The document outlines the standard operating procedures (SOPs) in pharmaceutical industries, emphasizing their importance in ensuring quality control and compliance with regulations. It details the structure, writing style, and various types of SOPs necessary for operational consistencies, such as Master Batch Records and Batch Manufacturing Records. The document also discusses the roles and responsibilities involved in SOP development, review, and implementation to ensure effective training and safety in procedures.