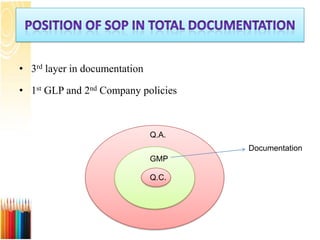
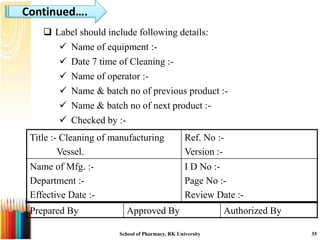
This document provides an introduction to a seminar on standard operating procedures (SOPs) for manufacturing liquid and semi-solid dosage forms. It discusses what an SOP is, the objectives and benefits of SOPs, different types of SOPs, how to write an SOP, common equipment used in liquid and semi-solid preparation, and provides examples of general SOPs.