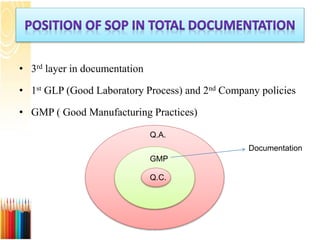
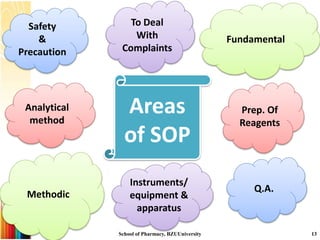
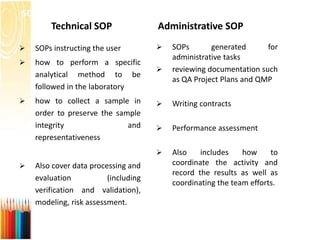
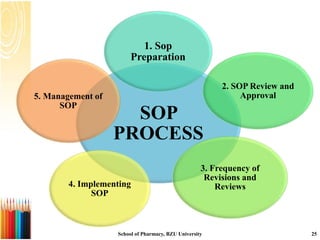
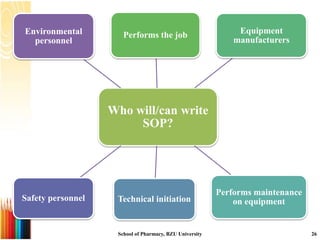
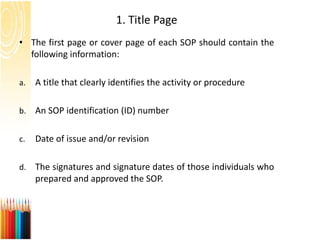
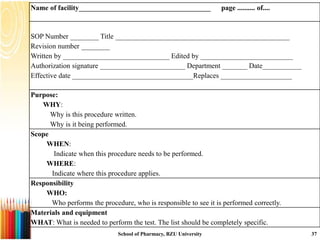
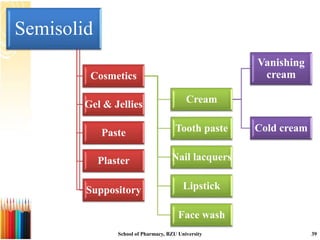
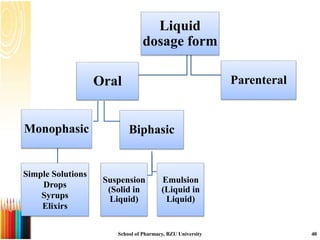
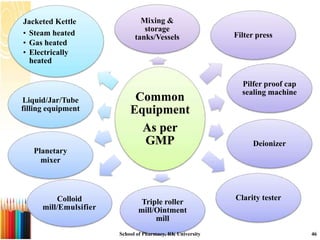
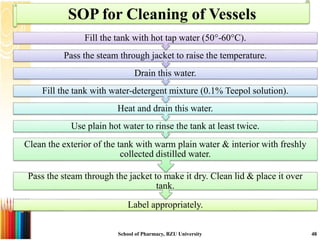
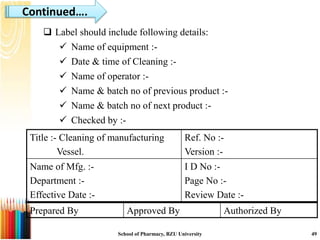
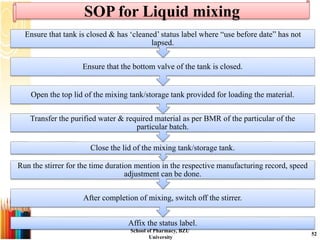
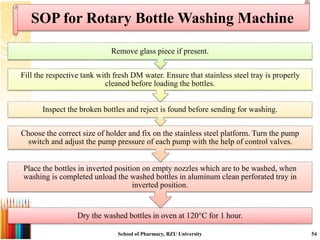
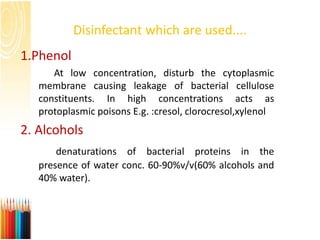
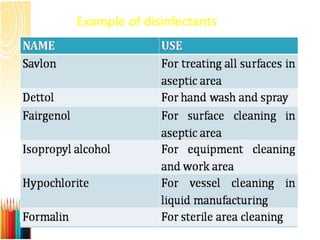
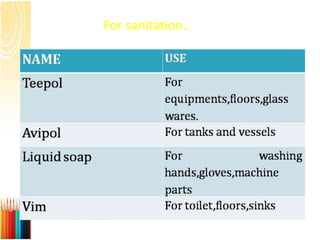
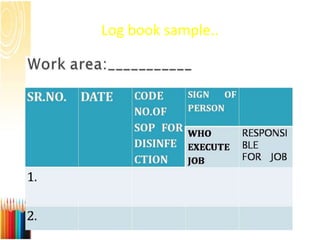
This document provides information on standard operating procedures (SOPs) for manufacturing equipment used in liquid, solid, and semi-solid dosage forms. It discusses what an SOP is, the objectives and benefits of SOPs, types of SOPs, SOP writing style, important points to consider when writing SOPs, and common equipment used to prepare liquid and semi-solid formulations. The document emphasizes that SOPs are essential for ensuring consistent, compliant production operations and for training personnel. They help maintain quality control and assure regulatory compliance.