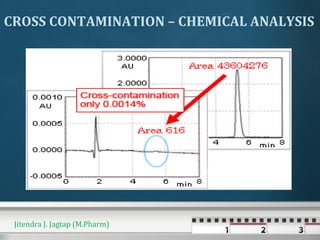
The document discusses cross contamination in pharmaceutical manufacturing. It states that the manufacturing environment is critical for product quality and can impact light, temperature, humidity, air movement and microbial and particulate contamination. Poorly designed or maintained air handling systems, inadequate cleaning procedures, and insufficient personnel and equipment procedures can lead to cross contamination originating from the environment, operators or equipment. Cross contamination can be minimized through skilled personnel, adequate facility design, closed production systems, validated cleaning procedures, and appropriate air pressure differentials in heating, ventilation and air conditioning systems.