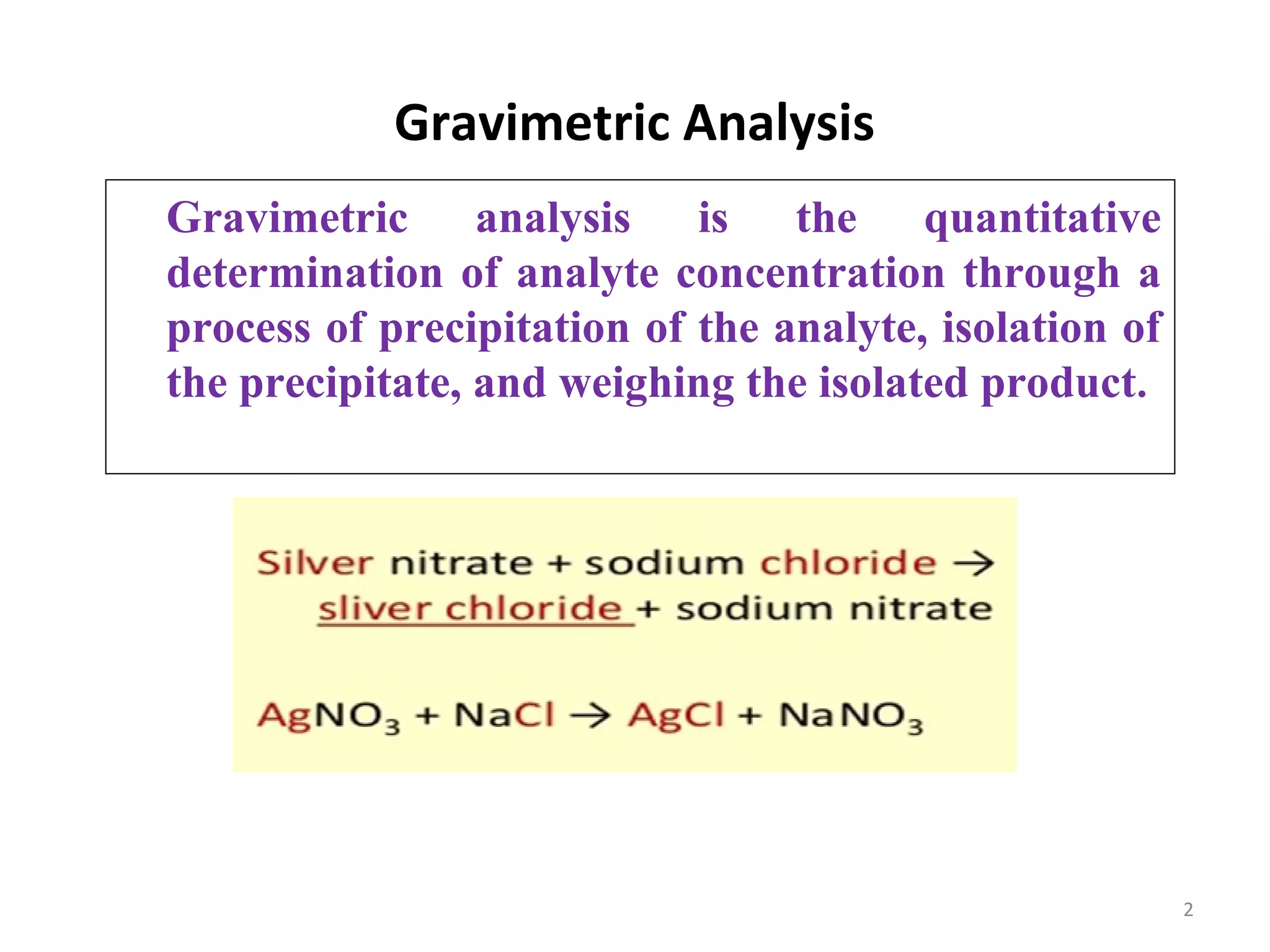
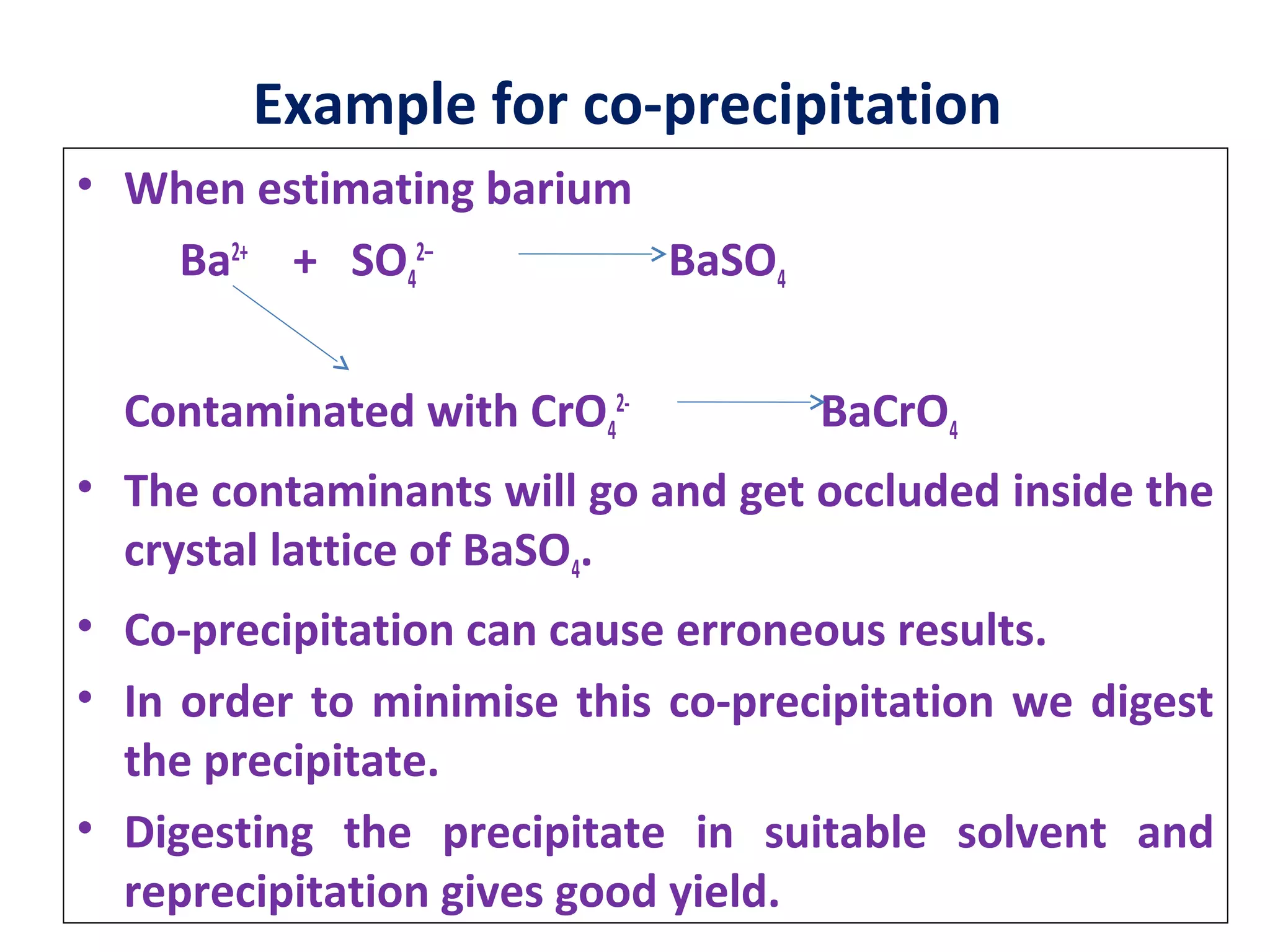
This document discusses gravimetric analysis, which is a quantitative analytical chemistry technique. It involves precipitating the analyte out of solution, isolating the precipitate, and weighing it. Key steps include dissolving a weighed sample, adding excess precipitating agent, filtering and drying the precipitate, and determining the original ion amount from the precipitate mass and composition. Crucibles made of porcelain or silica are used to dry precipitates in an oven, while sintered crucibles are used to dry in air. Contamination can occur via co-precipitation or post-precipitation of impurities. Gravimetric factors relate precipitate masses to analyte masses. Solubility of precipitates is affected by temperature,