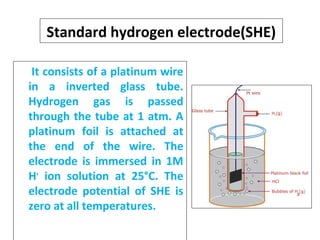
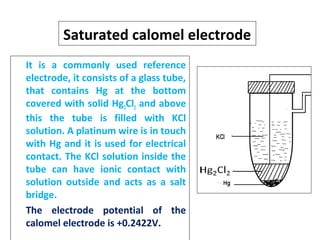
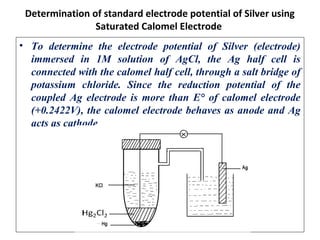
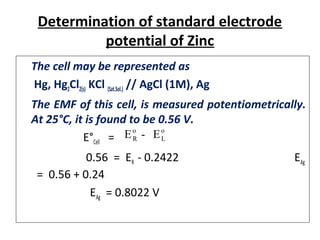
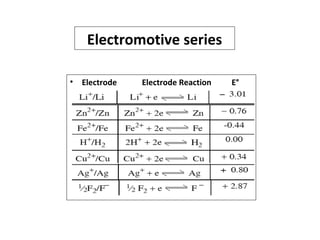
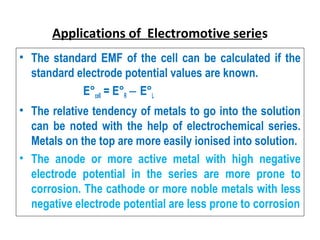
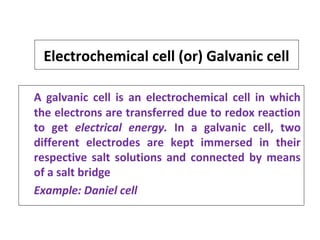
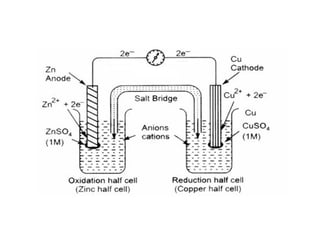
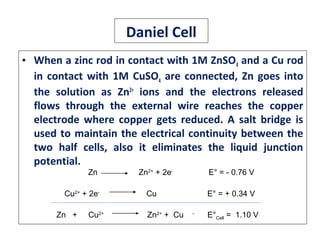
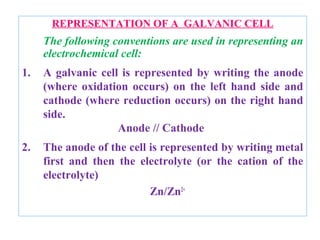
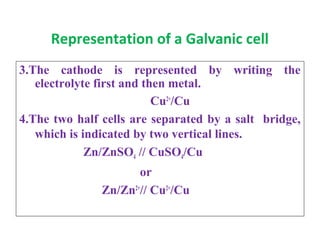
The document discusses electrode potential, which describes how metals behave in solutions of their own salts, detailing oxidation and reduction processes. It explains the standard hydrogen electrode as a reference for measuring electrode potentials and introduces the saturated calomel electrode as a secondary reference. Additionally, it covers electrochemical cells, particularly galvanic cells, illustrating how different metals interact in terms of oxidation and reduction, along with the use of the electromotive series to predict chemical reactions.

![Electrode potential
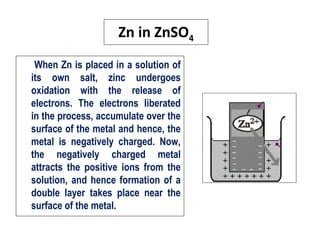
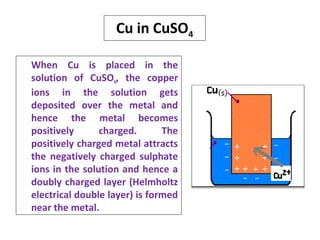
• The tendency of a metal to get oxidised or reduced when it
is placed in a solution of its own salt is called electrode
potential.
• When a metal [M] is placed in a solution containing its own
ions [Mn+
], then the metal may undergo either oxidation or
reduction. If the metal undergoes oxidation, then the
positive metal ions may pass into the solution
M Mn+
+ ne‒
• If the metal undergoes reduction, then the negative ions
may get deposited over the metal.
Mn+
+ ne‒
M](https://image.slidesharecdn.com/electrodepotential-150610103454-lva1-app6891/85/Electrode-potential-2-320.jpg)