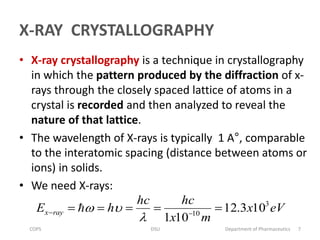
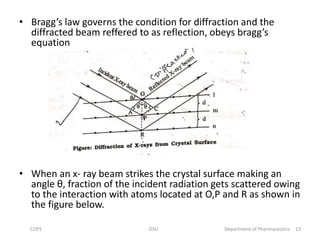
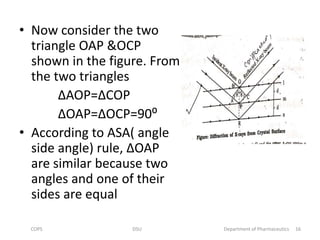
The document discusses x-ray crystallography, a technique used to analyze crystal structures by examining x-ray diffraction patterns. It covers the history of x-ray discovery, the properties and generation of x-rays, and details on Bragg's Law, which governs diffraction conditions. Important concepts include the interaction of x-rays with different atoms, constructive and destructive interference, and the unique diffraction patterns produced by different crystalline materials.