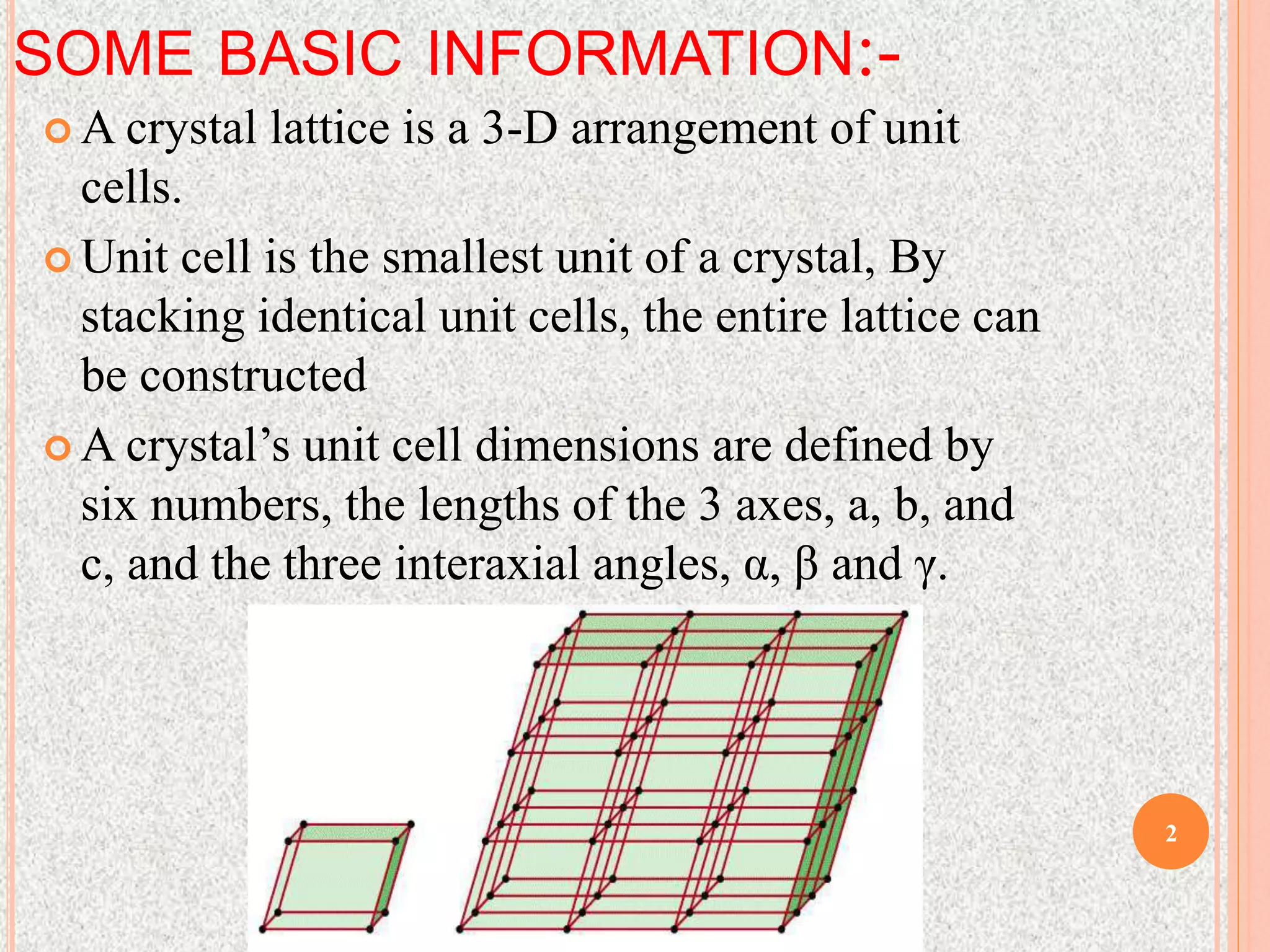
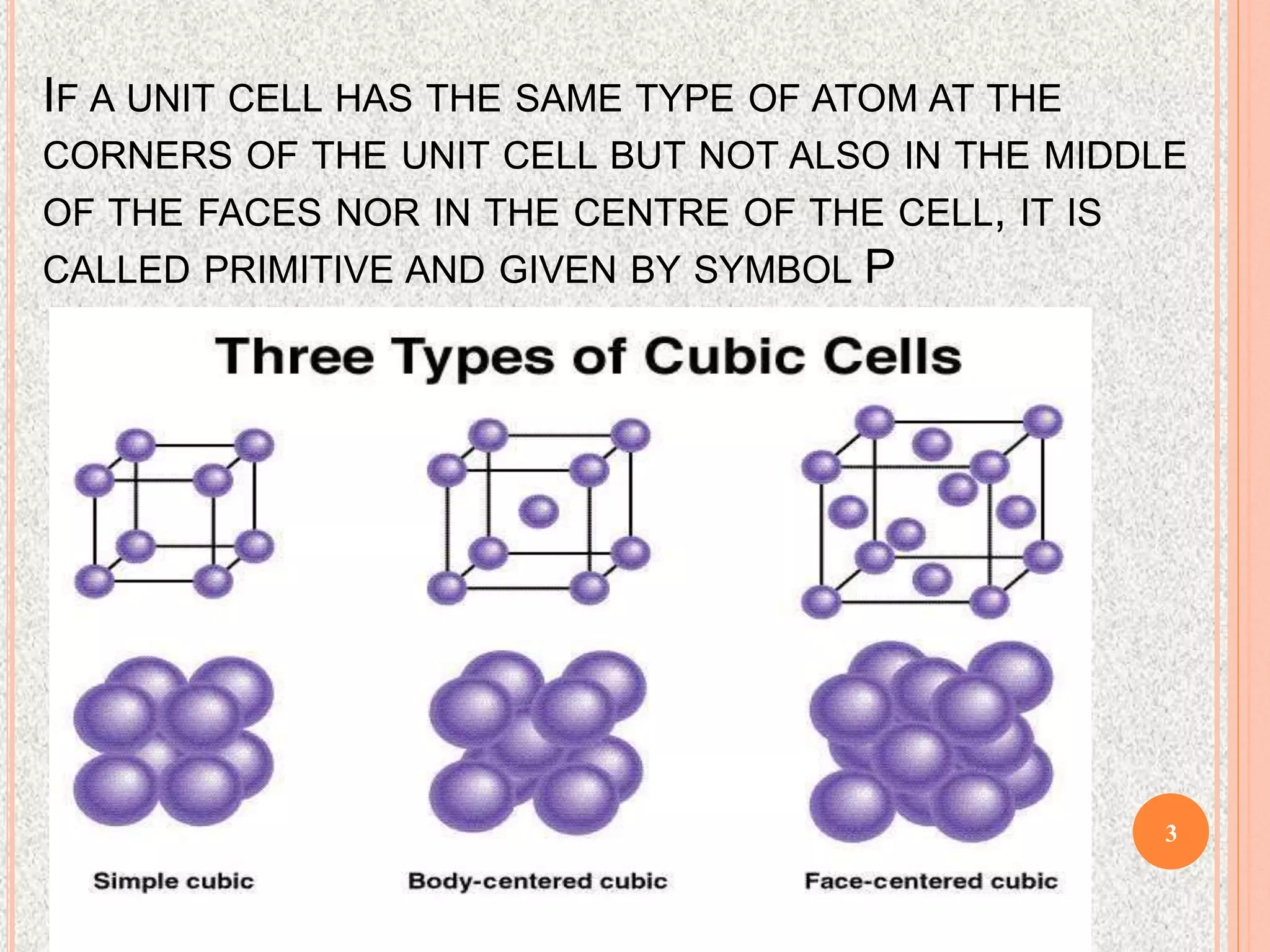
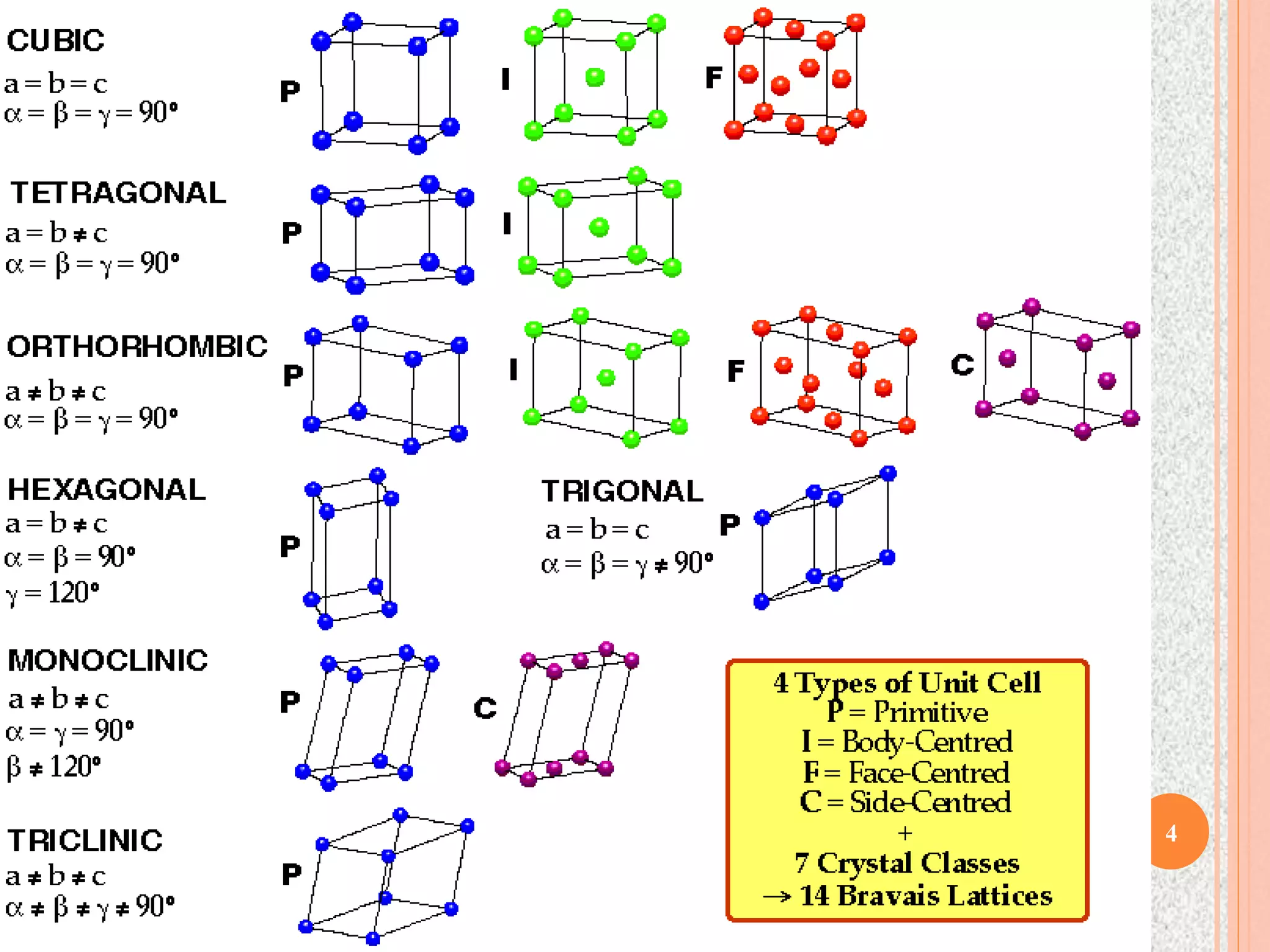
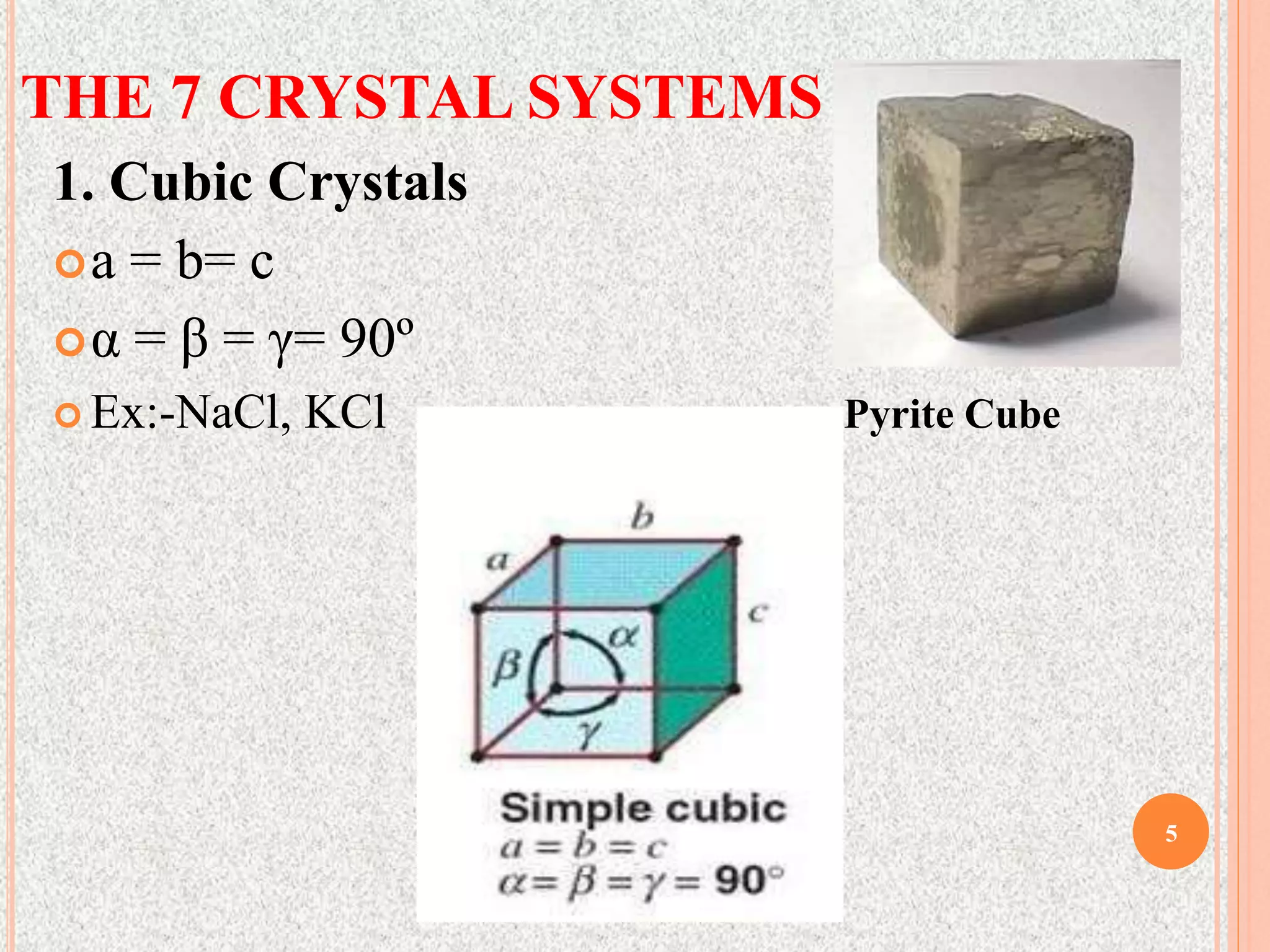
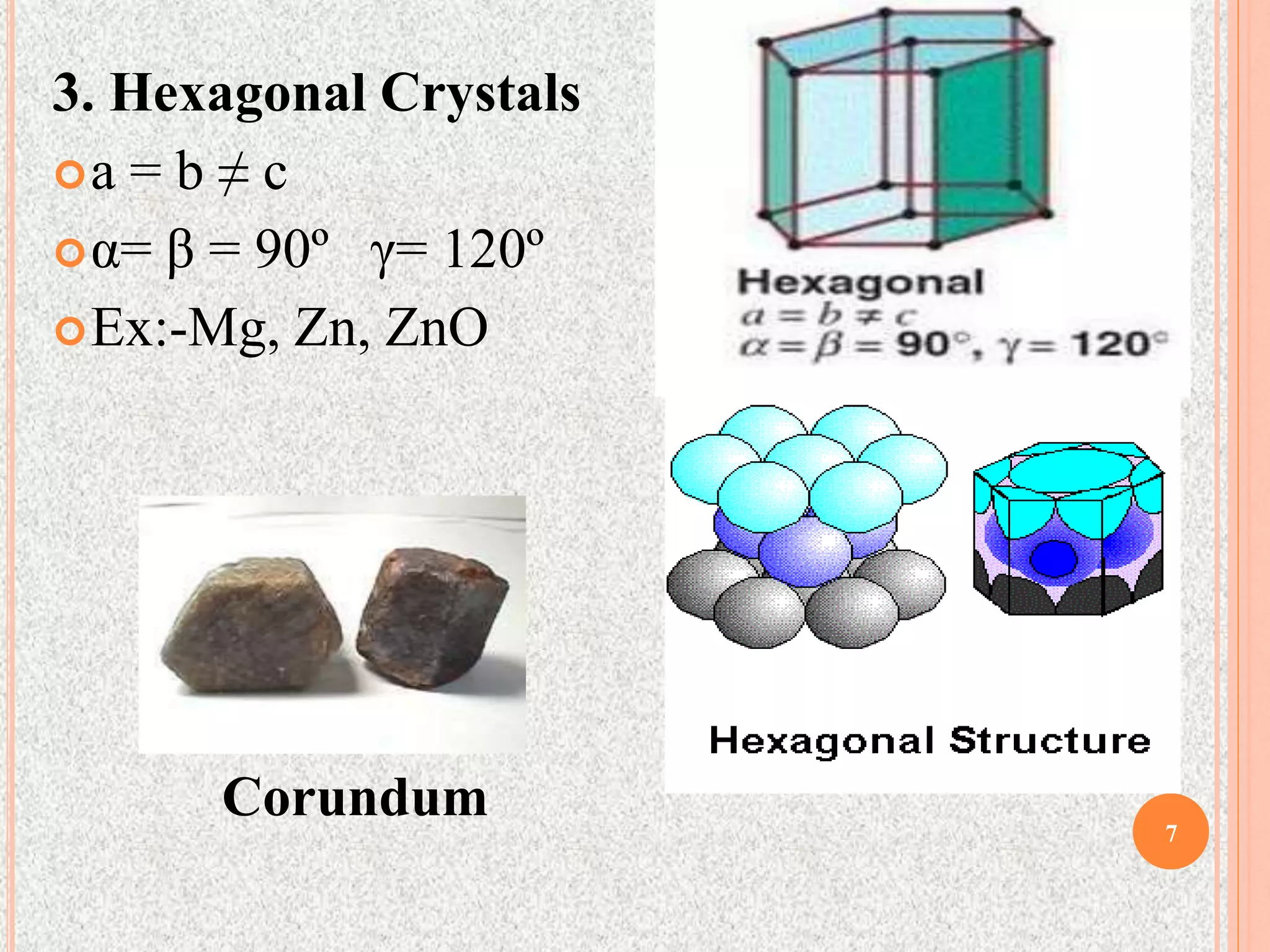
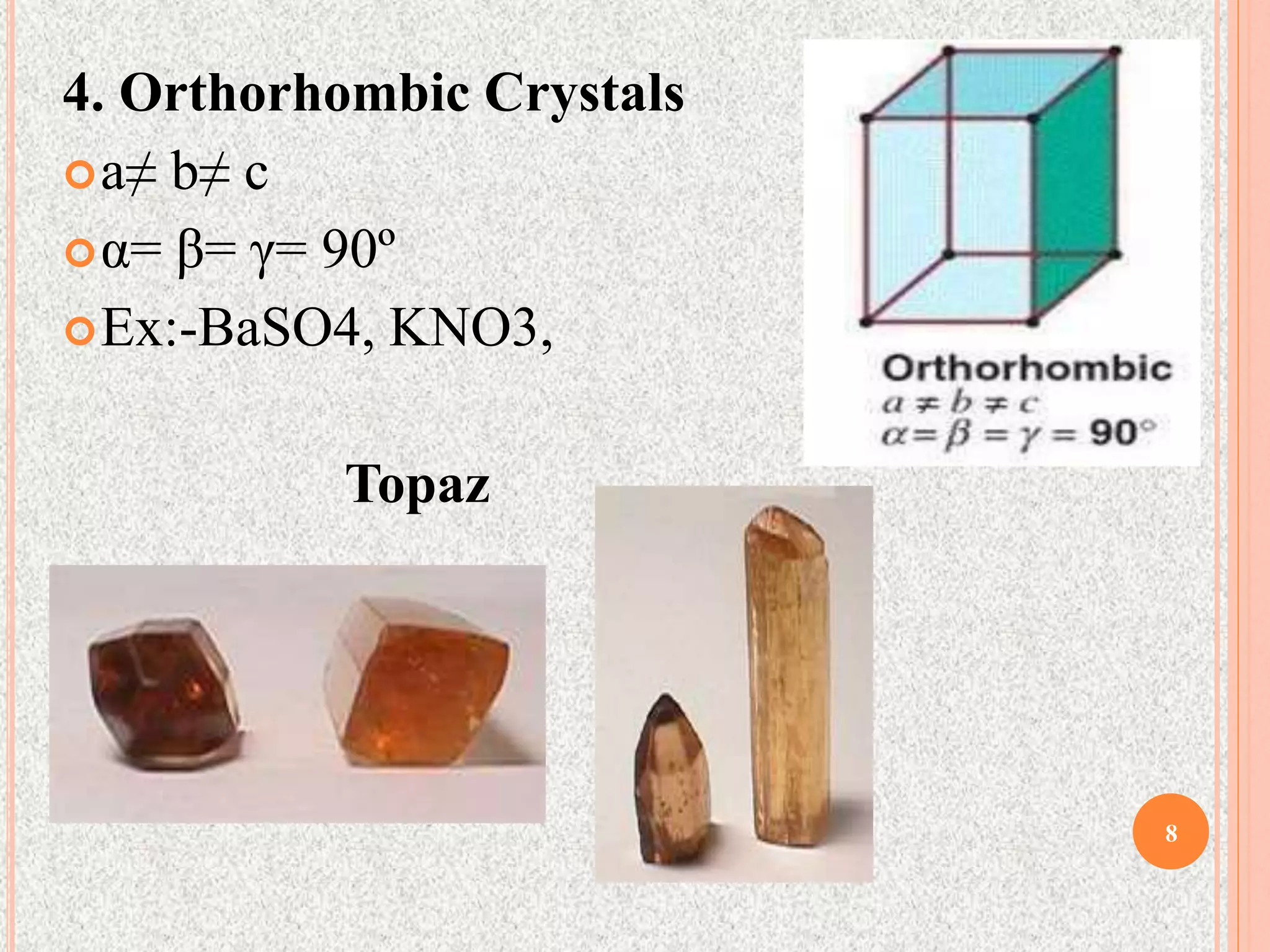
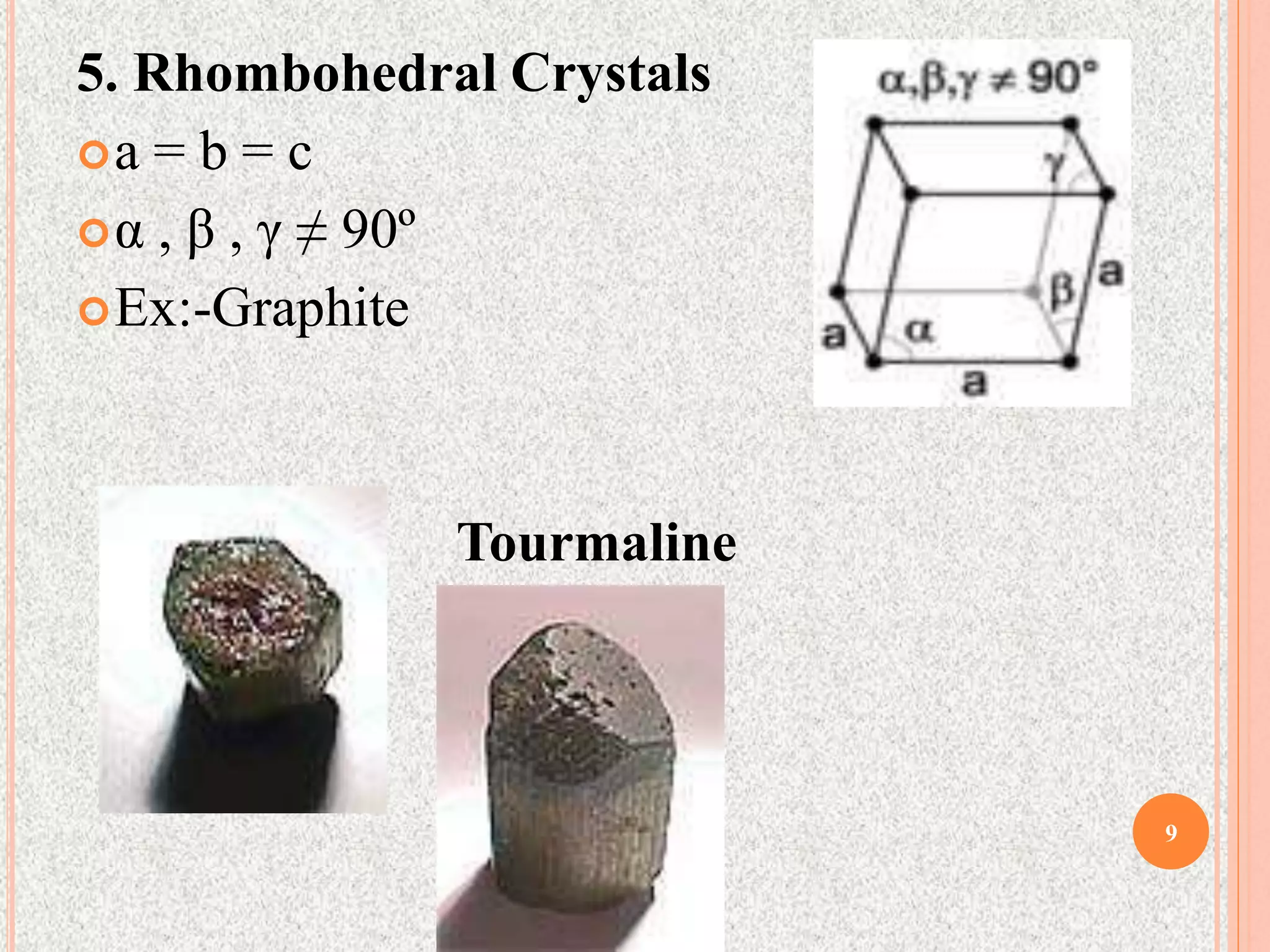
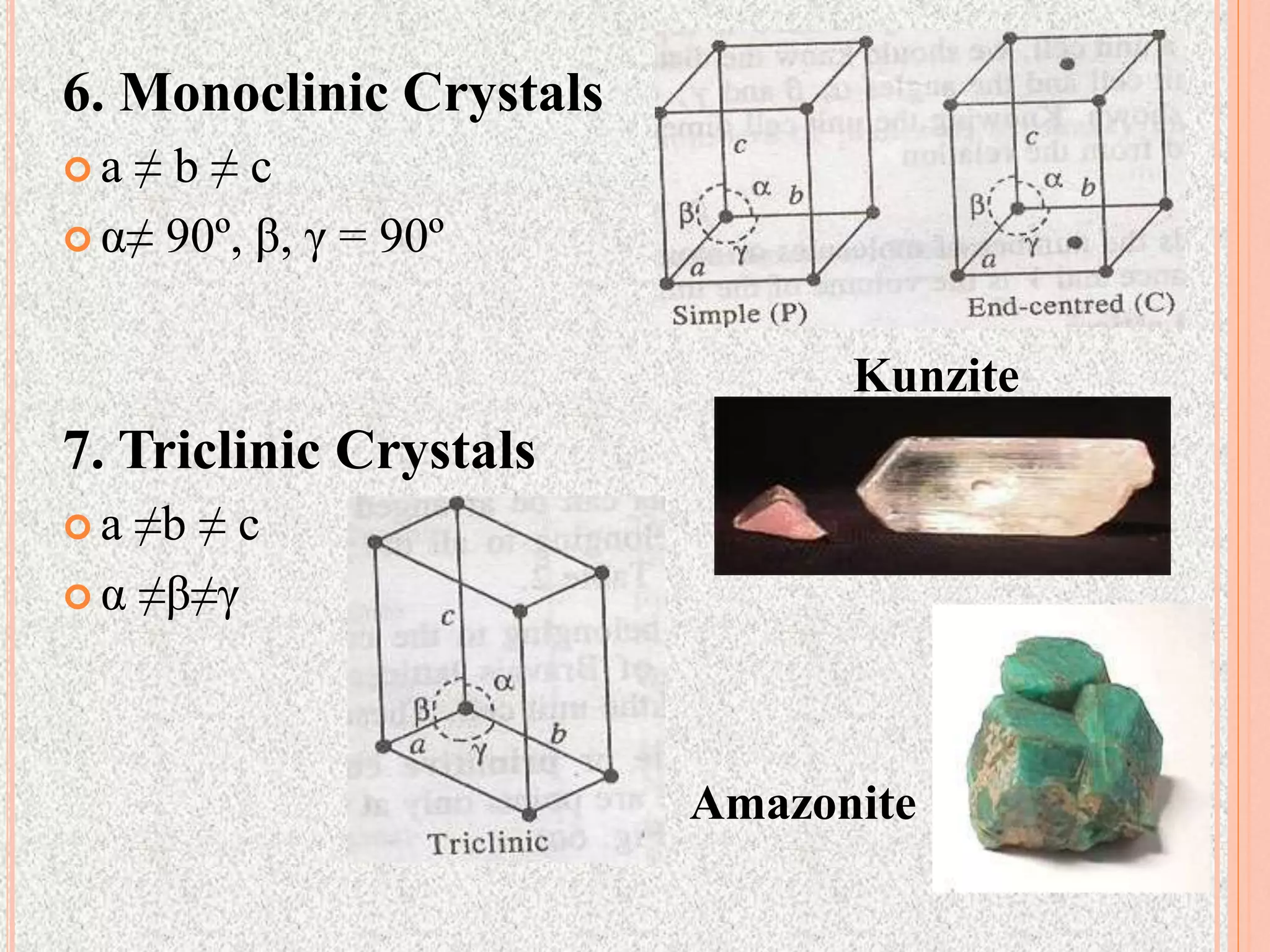
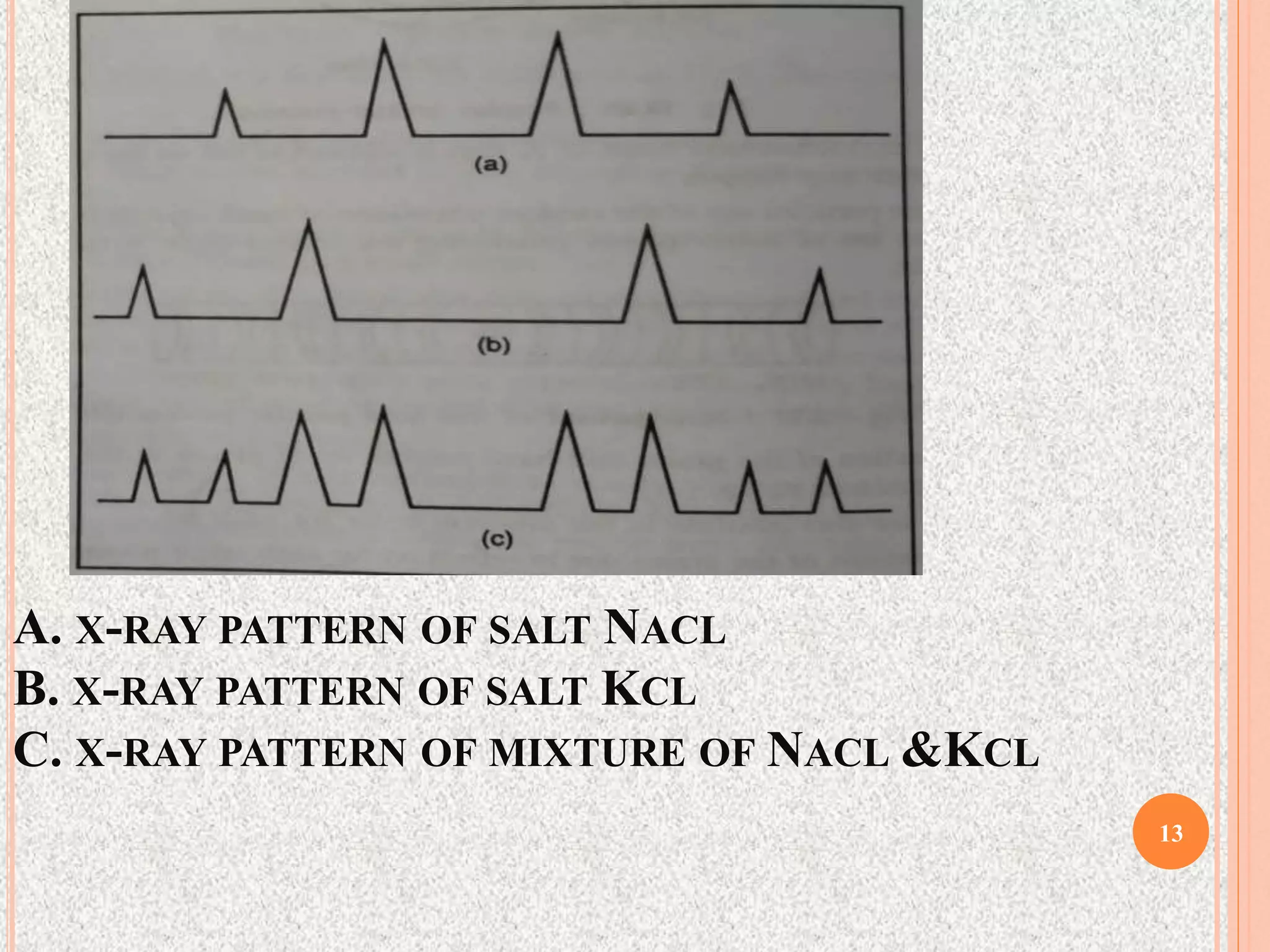
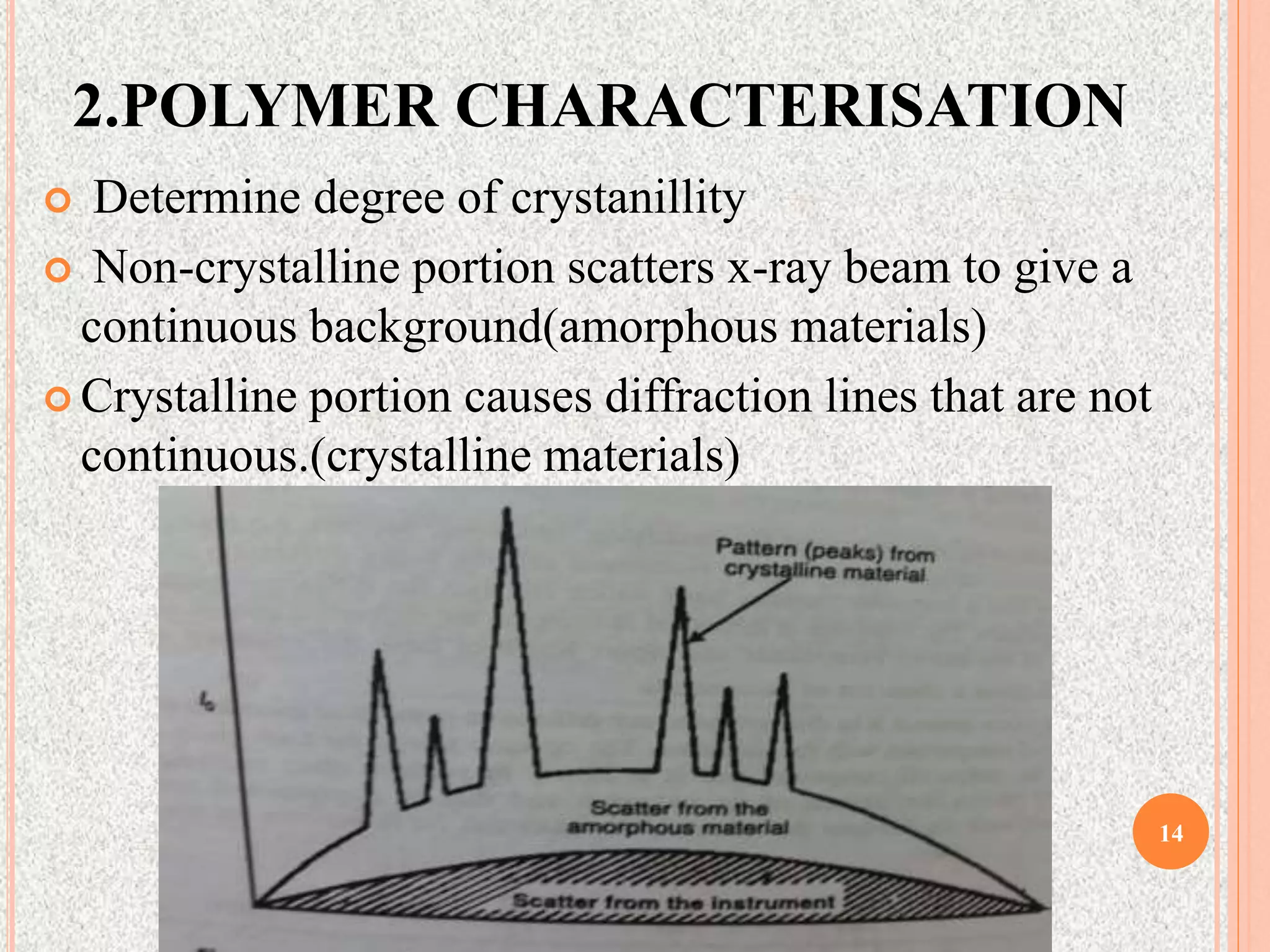
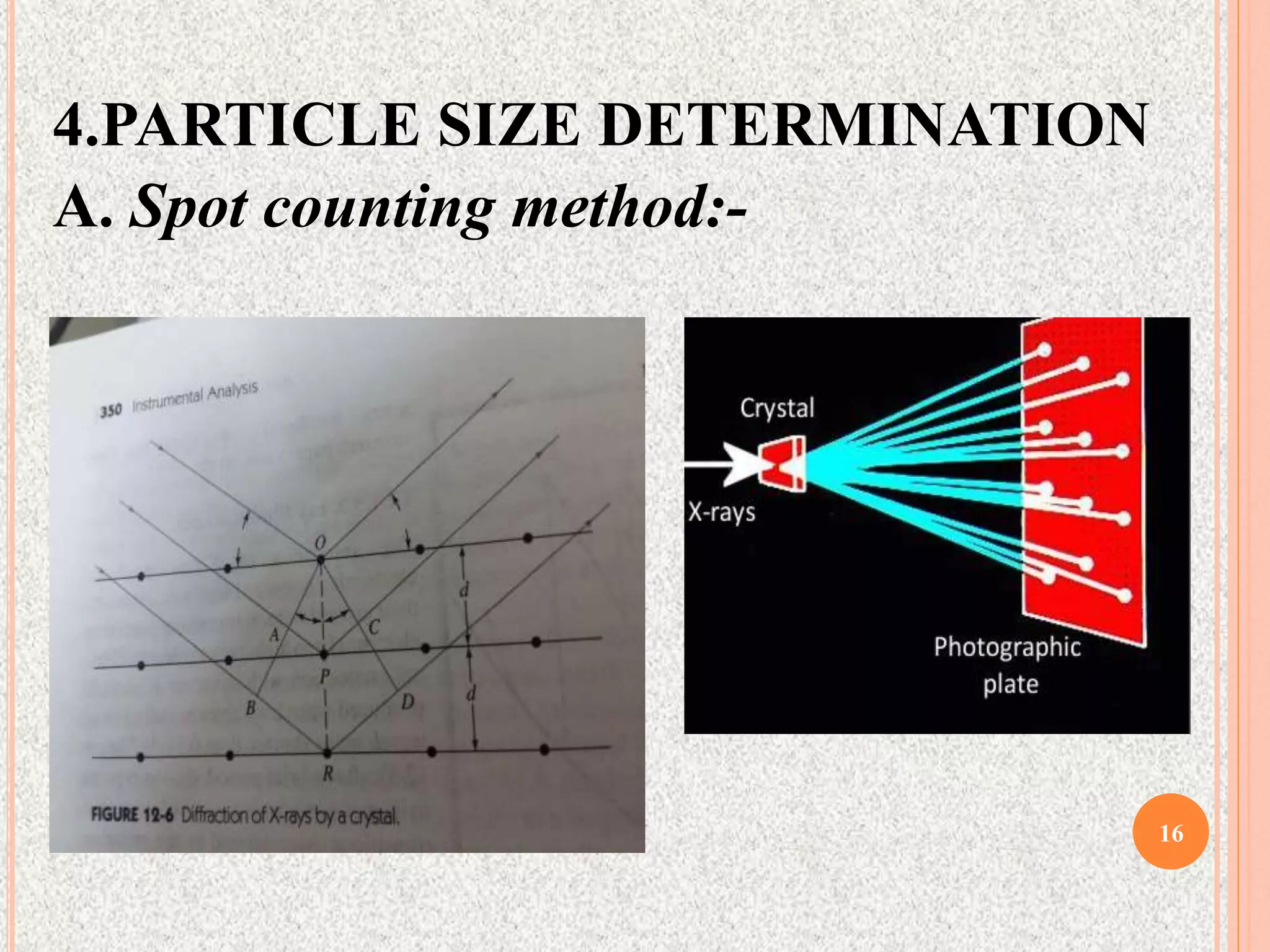
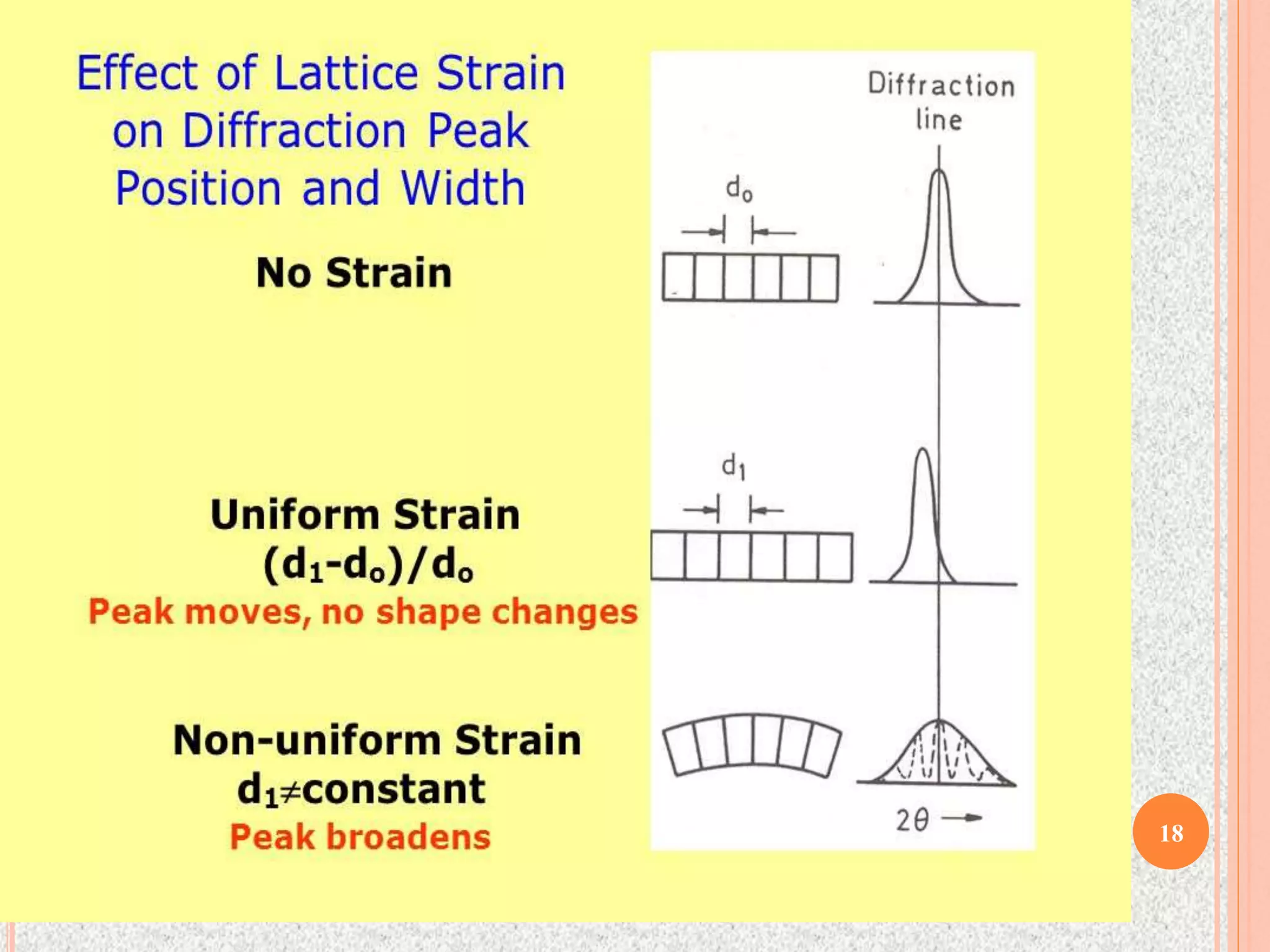
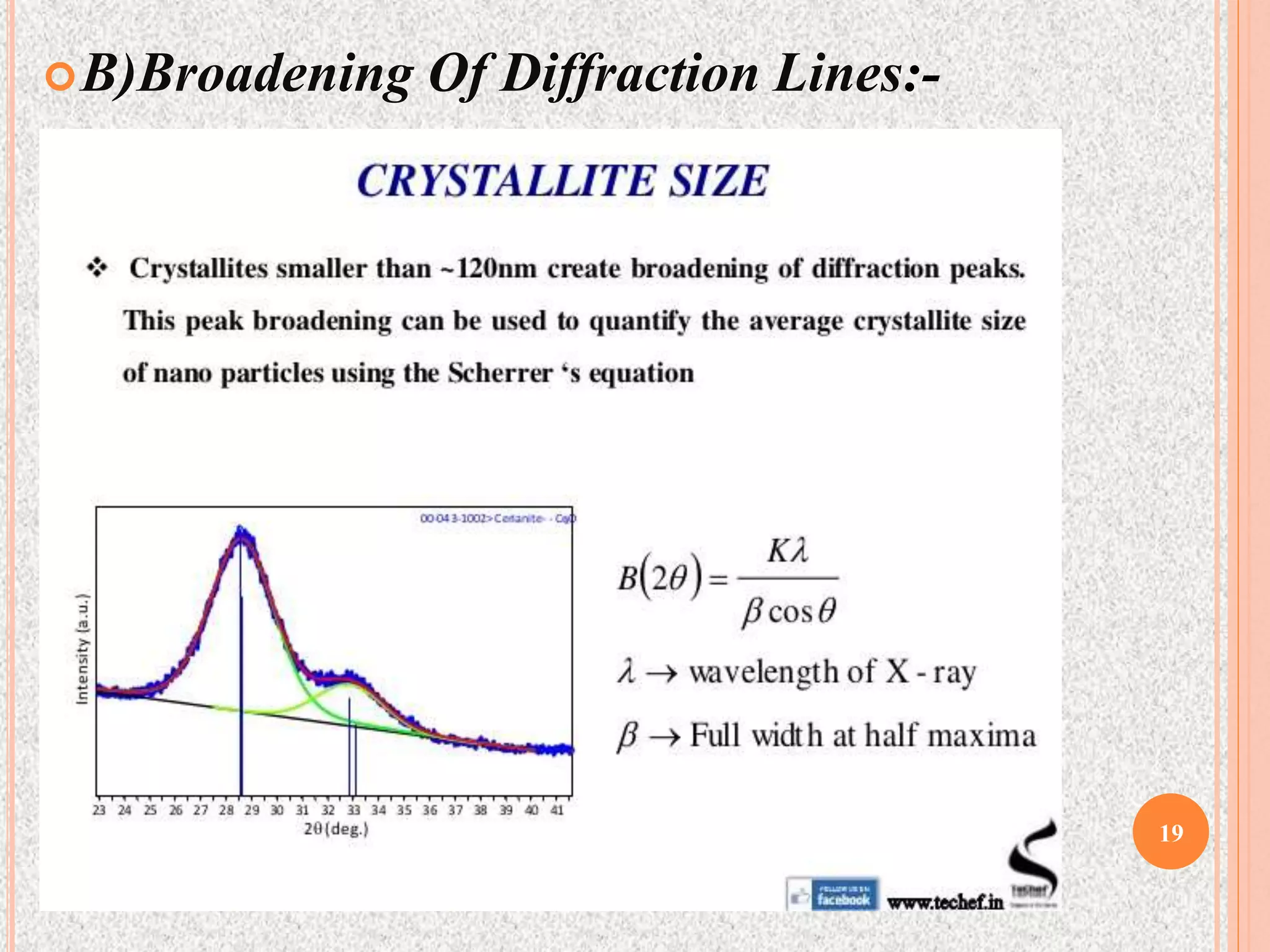
The document provides a comprehensive overview of x-ray diffraction, detailing crystal structures, unit cell definitions, and the seven types of crystal systems. It outlines the applications of x-ray crystallography in determining the structure of crystals, polymer characterization, and particle size determination, among others. Furthermore, it covers the significance of x-ray techniques in analyzing complex isomers and miscellaneous applications in various fields.